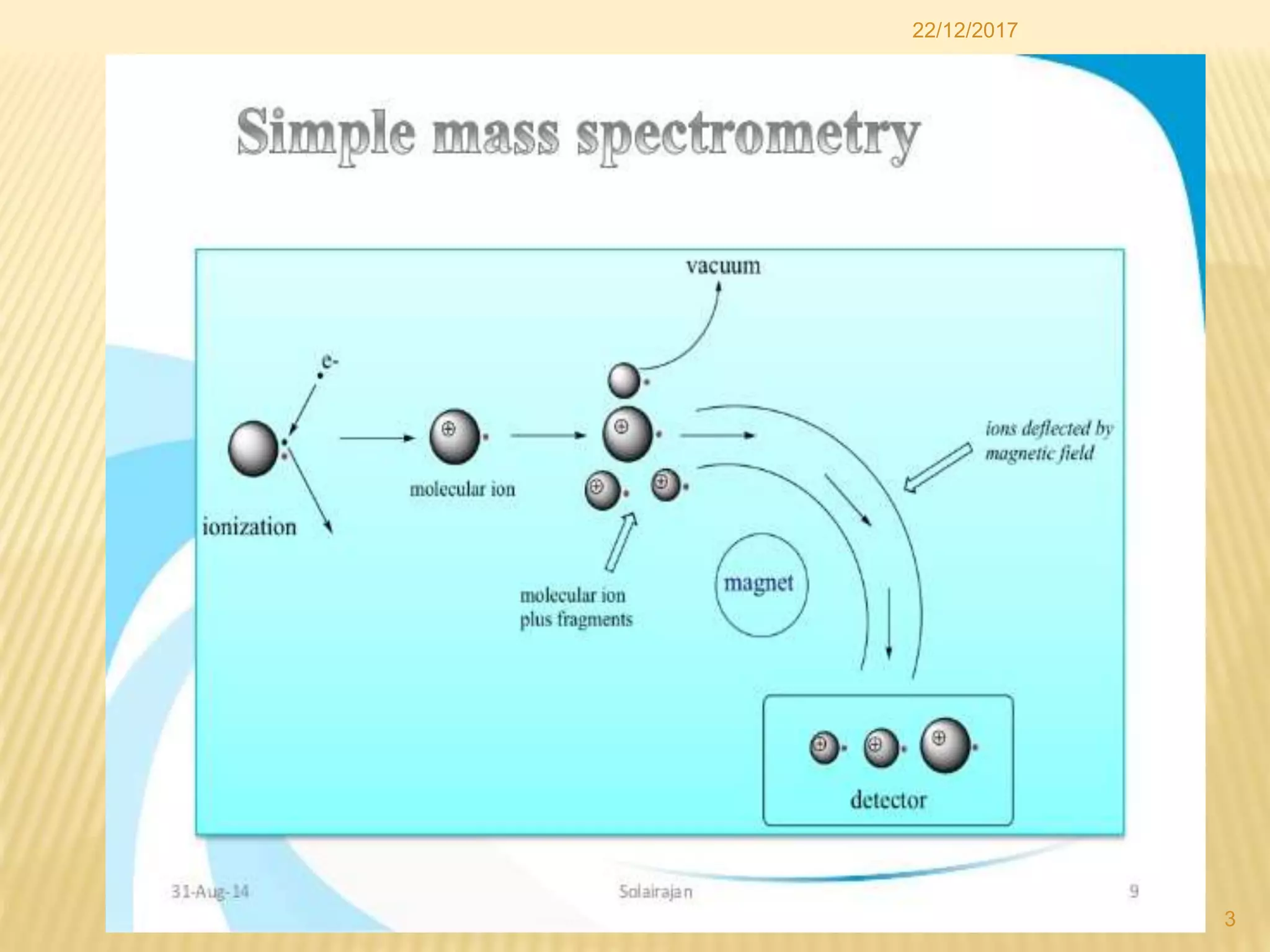
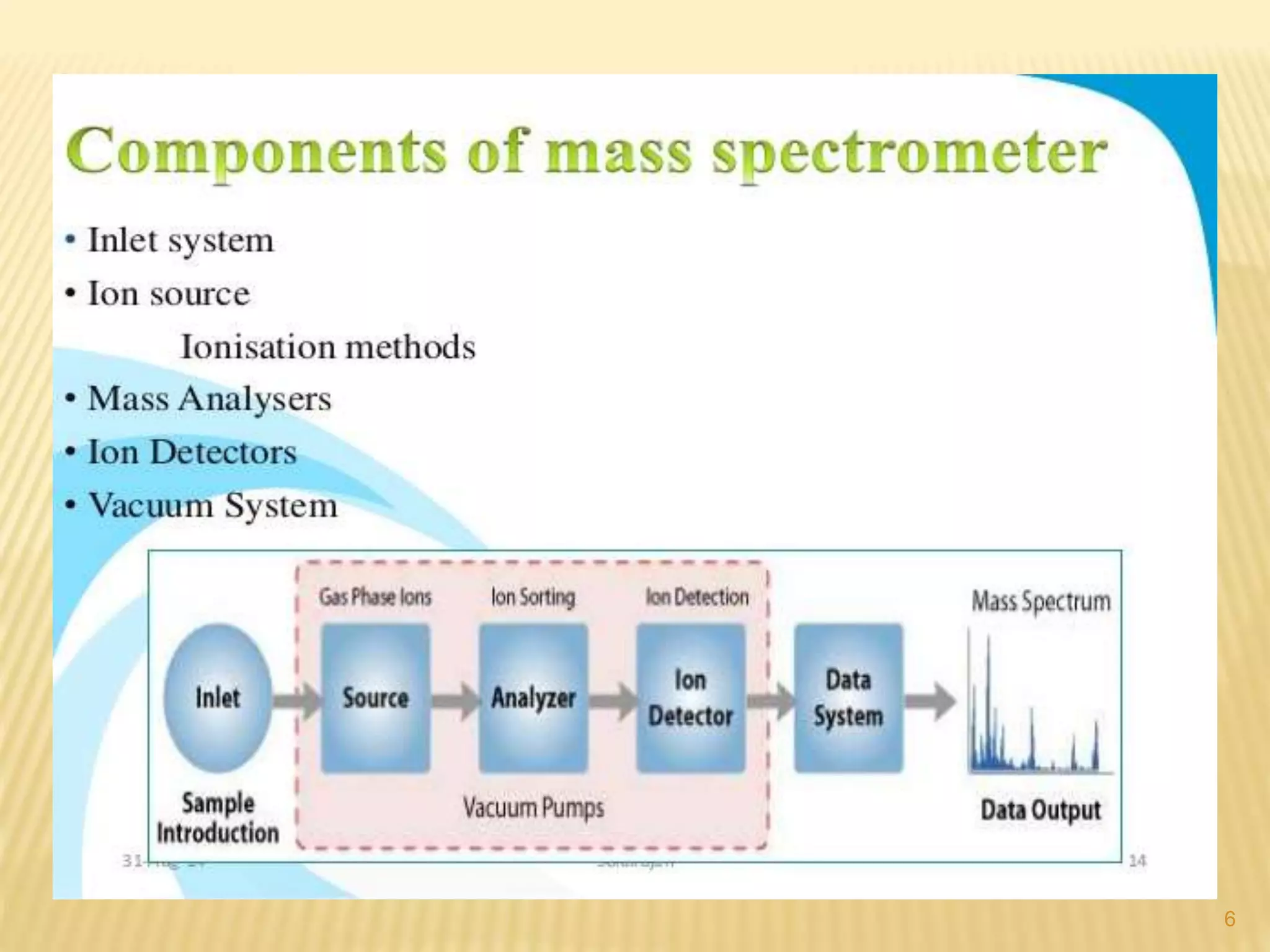
Mass spectrometry and gas chromatography are analytical chemistry techniques. Mass spectrometry identifies chemicals by measuring the mass-to-charge ratio of ions to determine molecular weights. Gas chromatography separates mixtures by vaporizing samples and carrying them through a column with an inert gas mobile phase. Different compounds migrate through the column at different rates and are detected to create a chromatogram. These techniques are useful for identifying compounds in mixtures like drugs, metabolites, fibers, and more.