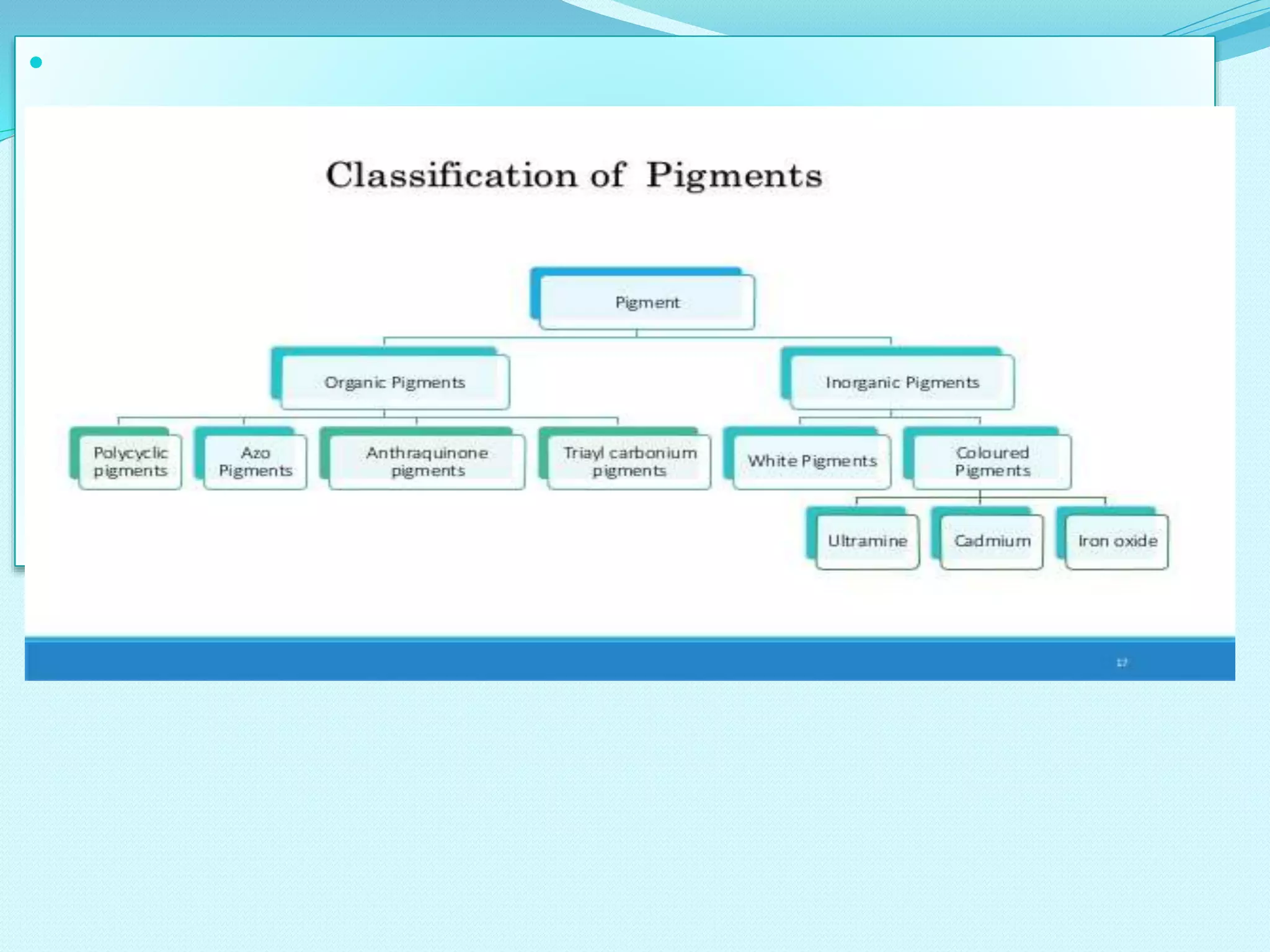
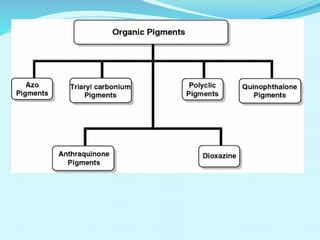
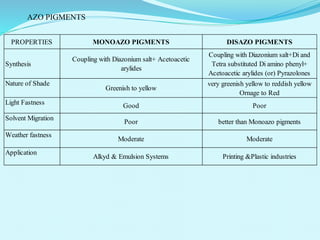
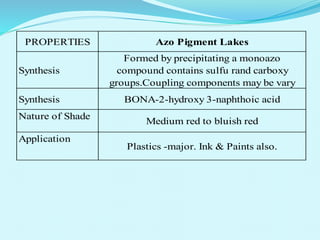
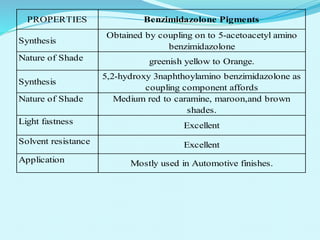
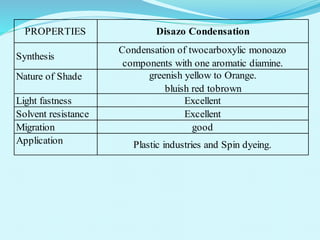
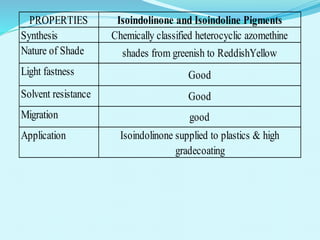
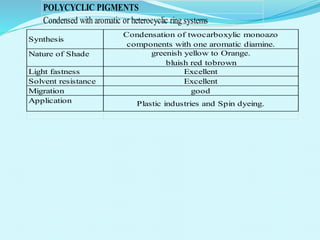
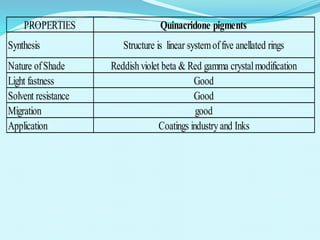
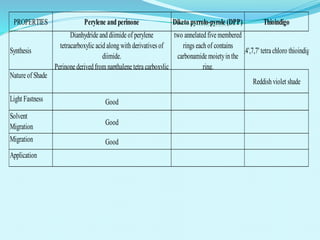
The document discusses various inorganic and organic pigments used in paints and coatings. It describes the chemical composition, synthesis, properties and applications of different classes of pigments including lead chromates, cadmium yellow, yellow oxides, bismuth vanadate, azo pigments, polycyclic pigments, anthraquinone pigments and others. Key pigments discussed are lead chromate, cadmium yellow, diarylide pigments, quinacridone, and metal complex pigments. The document provides information on properties like light fastness, opacity, and heat stability for selecting the right pigment.