This document discusses the physiological basis of coronary revascularization. It covers topics such as coronary physiology, myocardial viability assessment, and coronary revascularization. Some key points include:
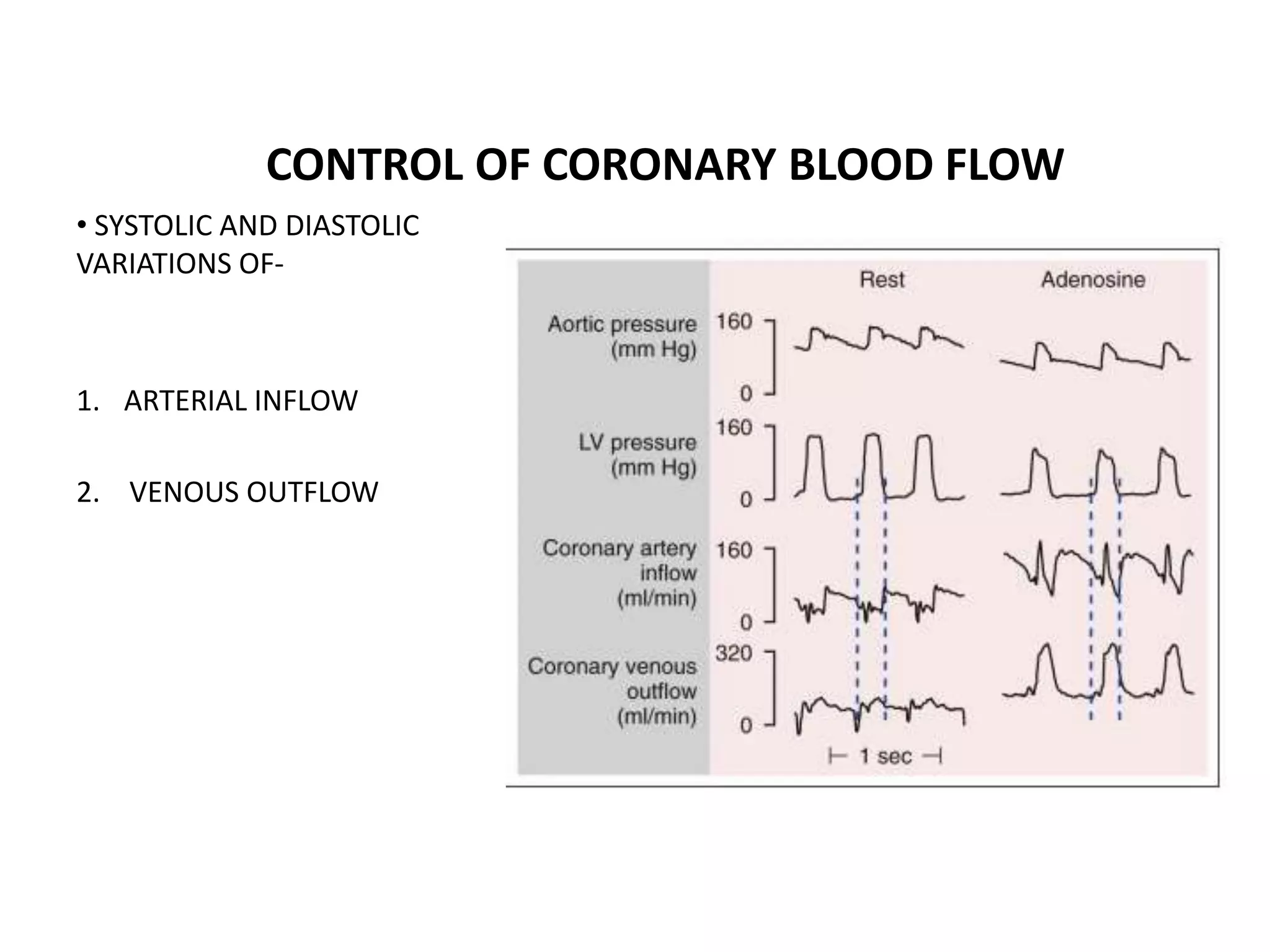
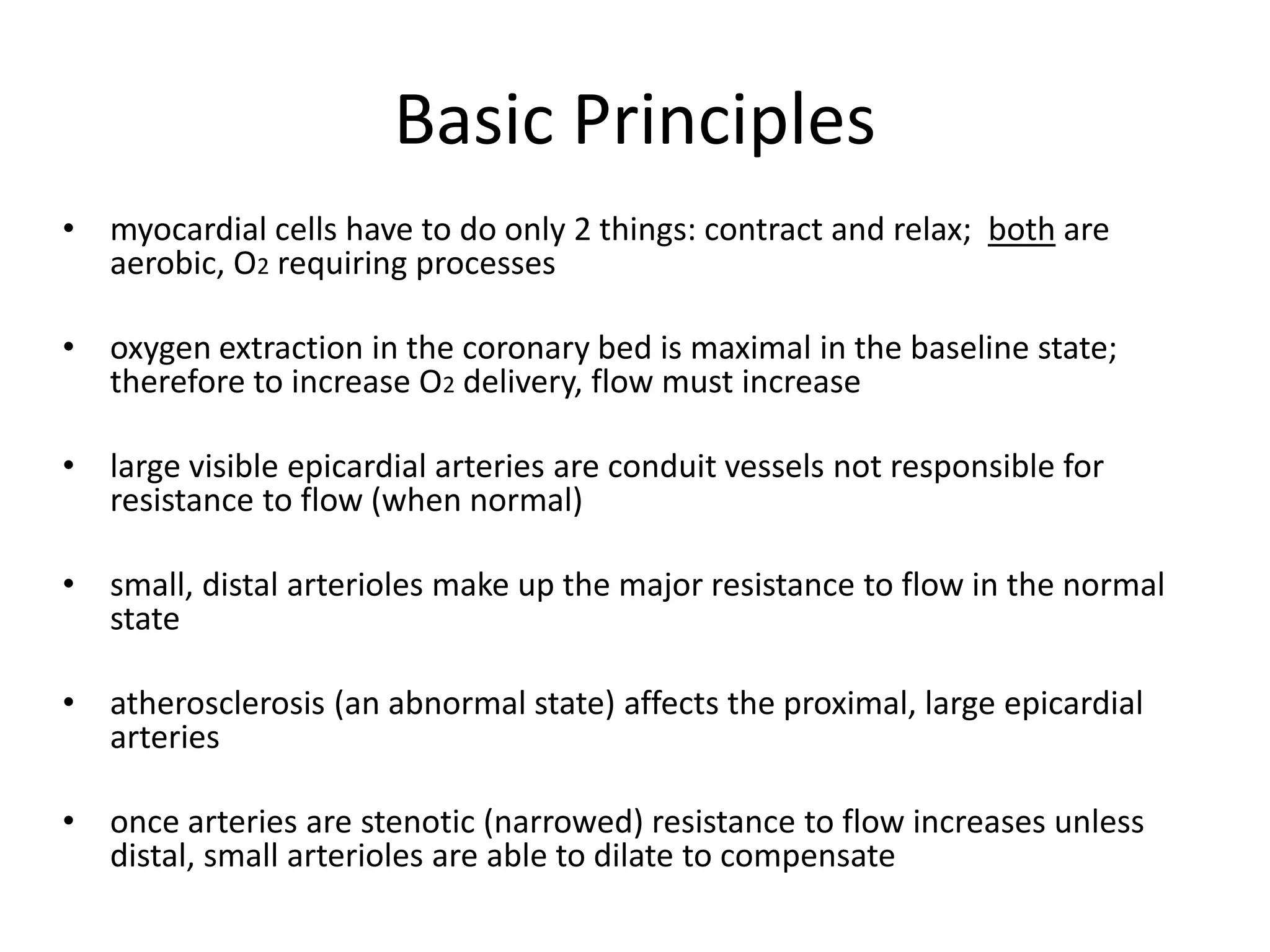
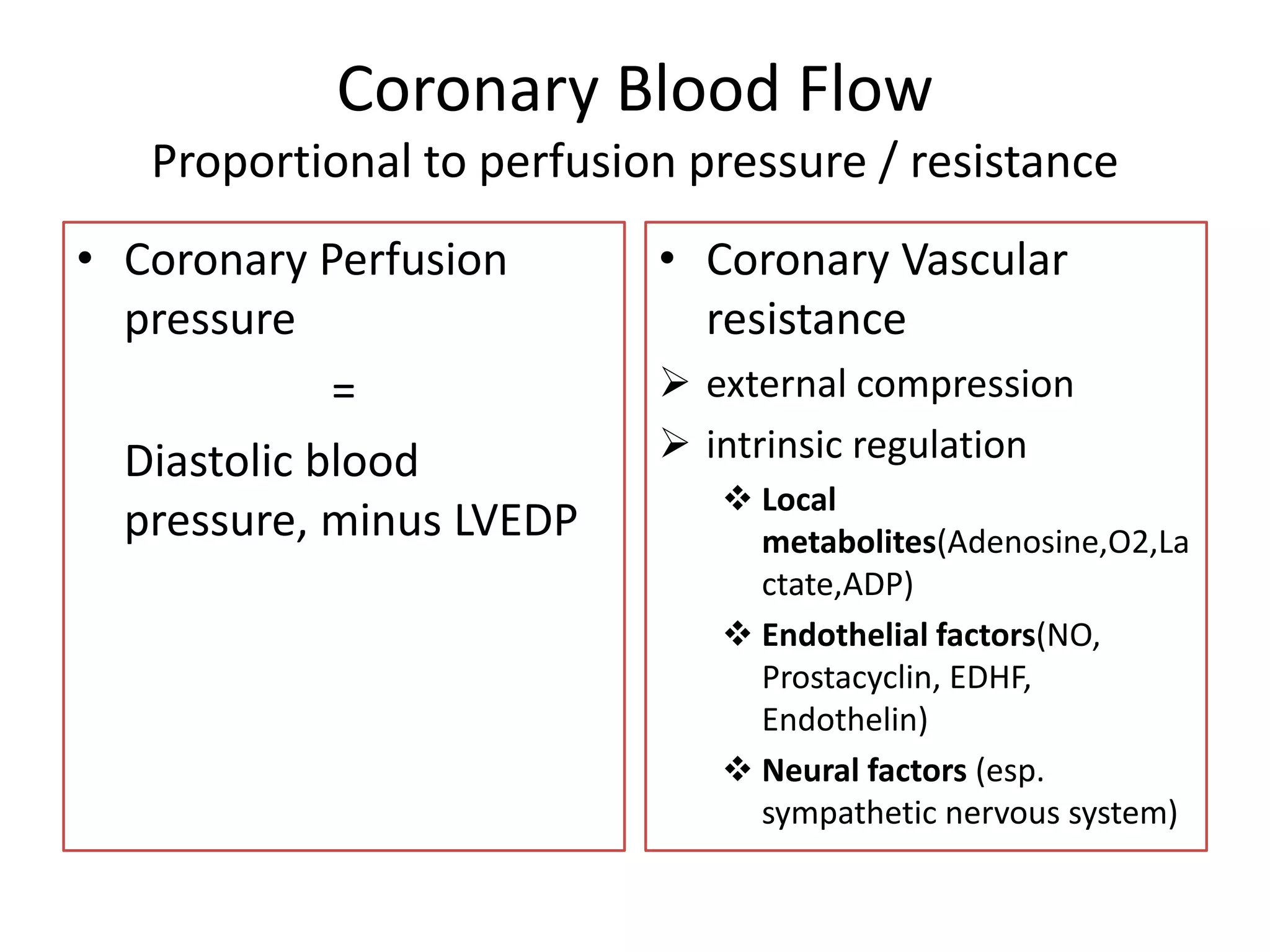
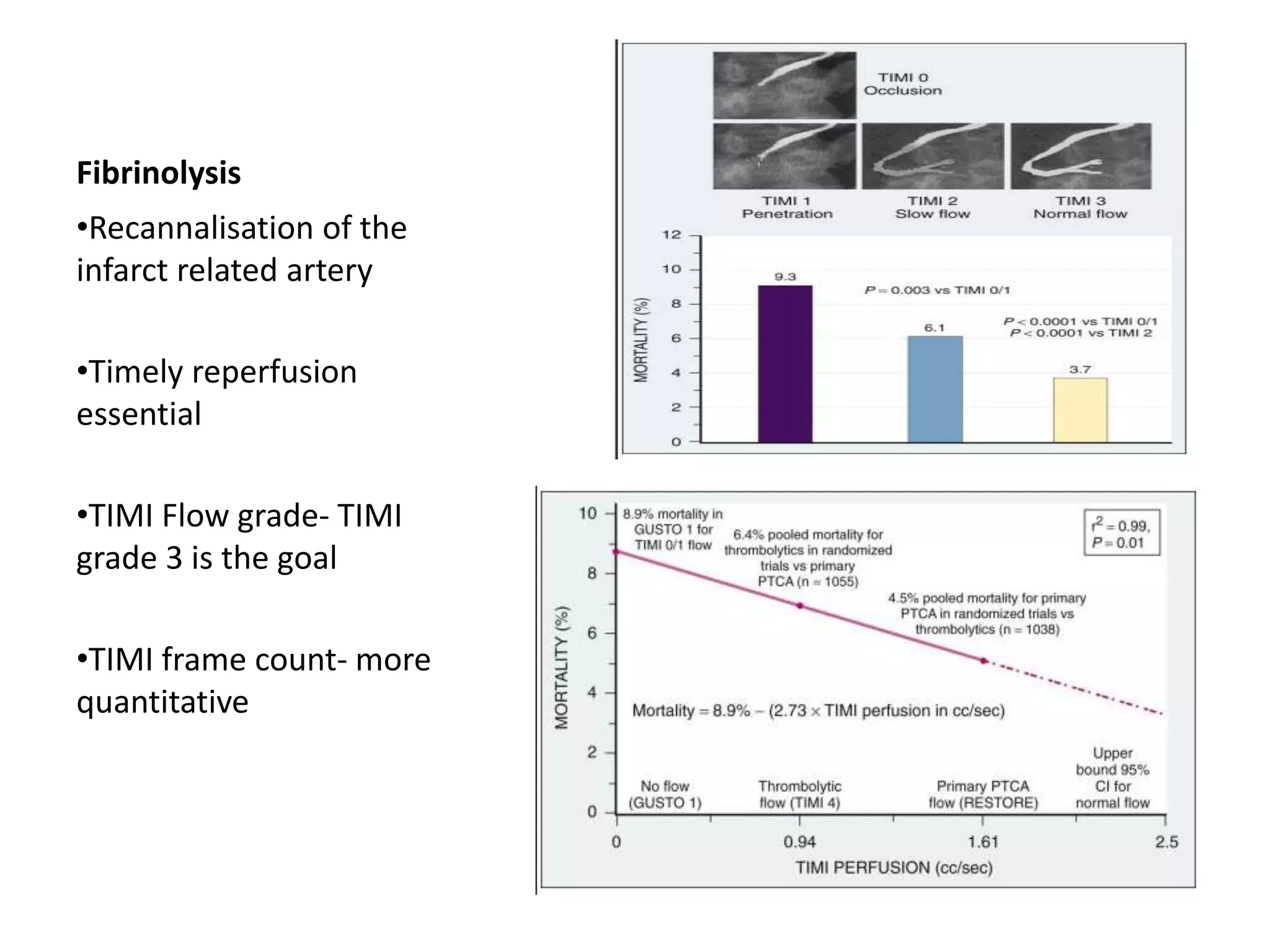
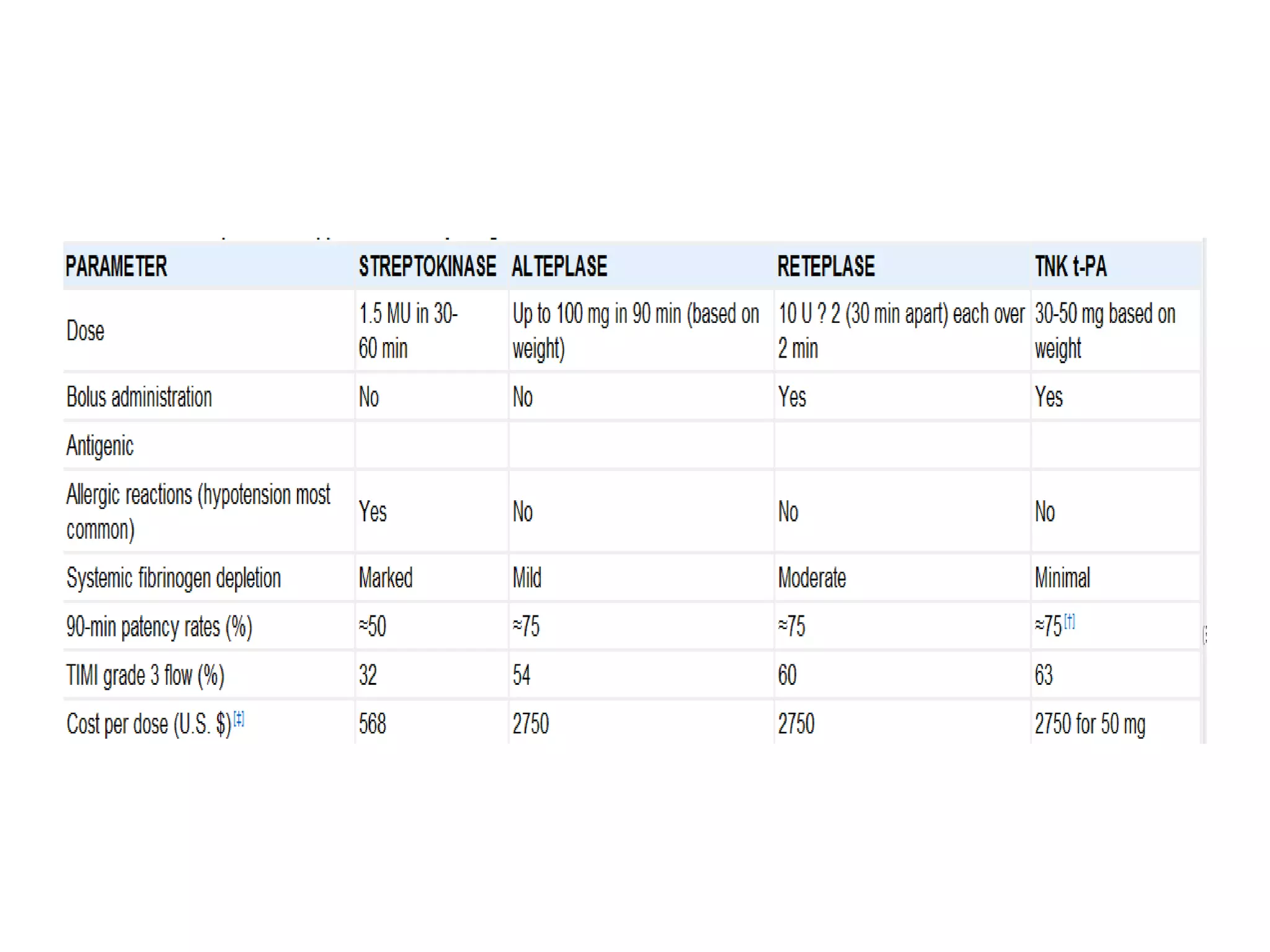
- Coronary blood flow is proportional to perfusion pressure over resistance and is regulated by various metabolic and endothelial factors.
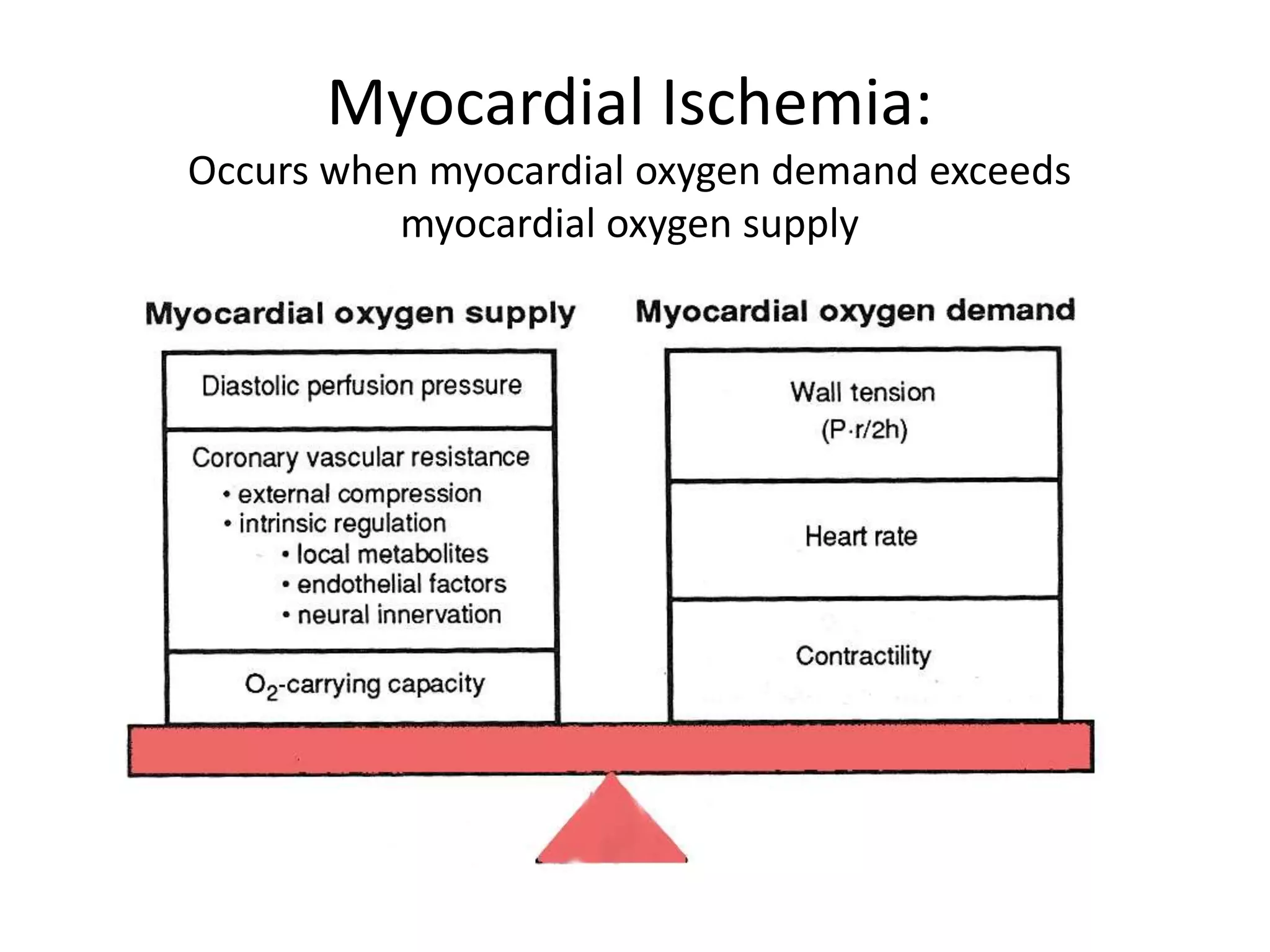
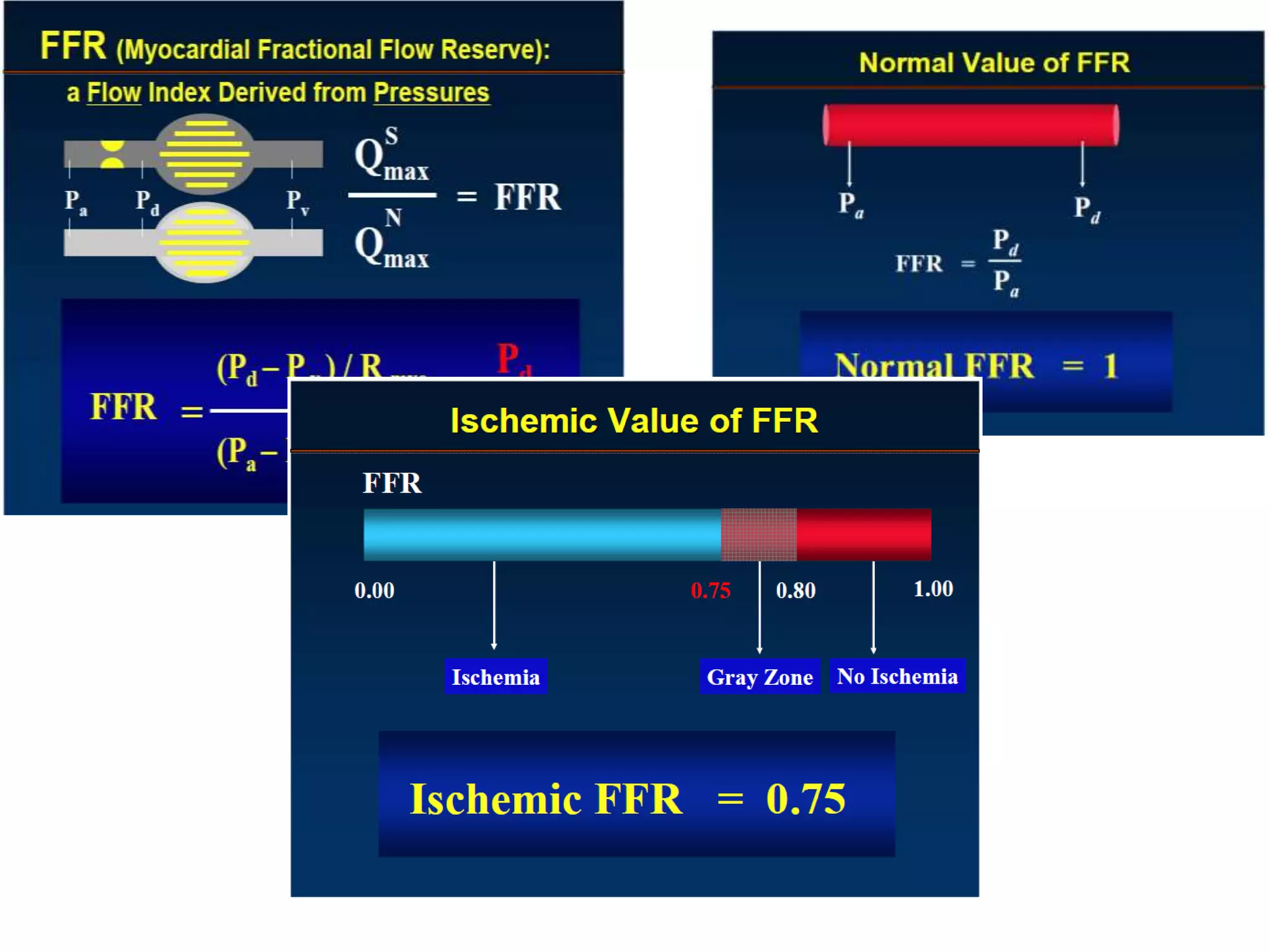
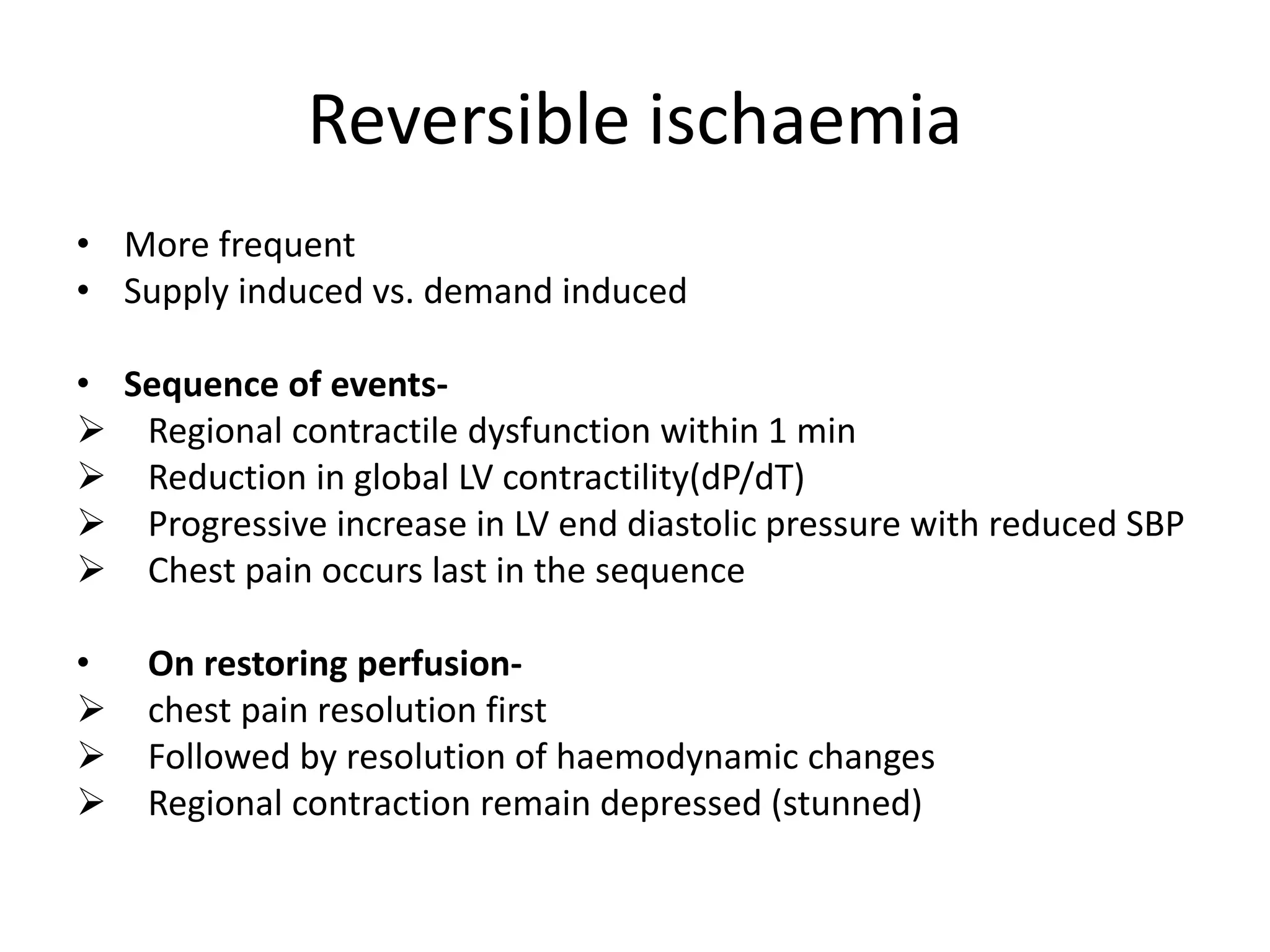
- Myocardial ischemia occurs when oxygen demand exceeds supply. Coronary autoregulation and flow reserve help maintain adequate flow.
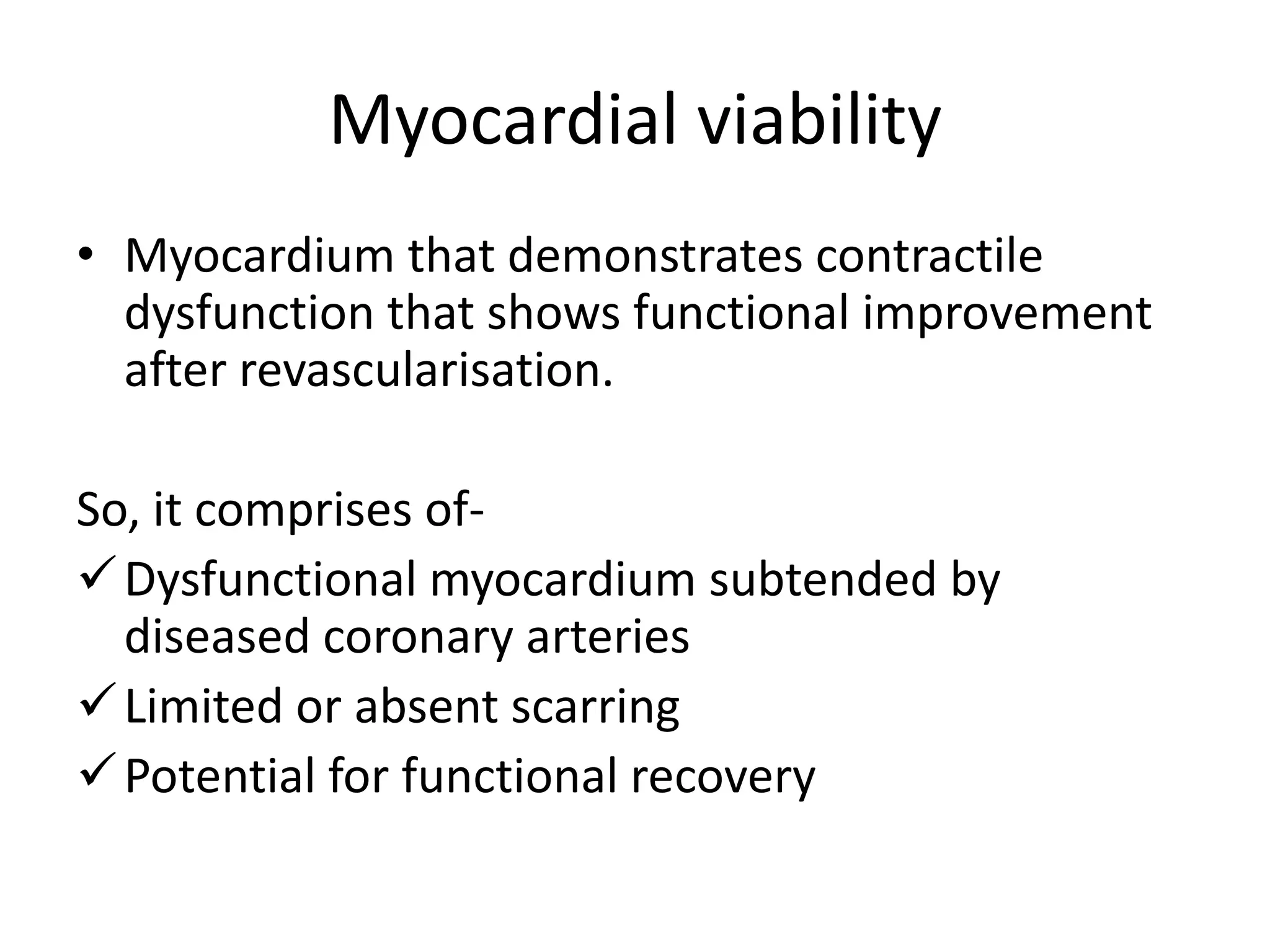
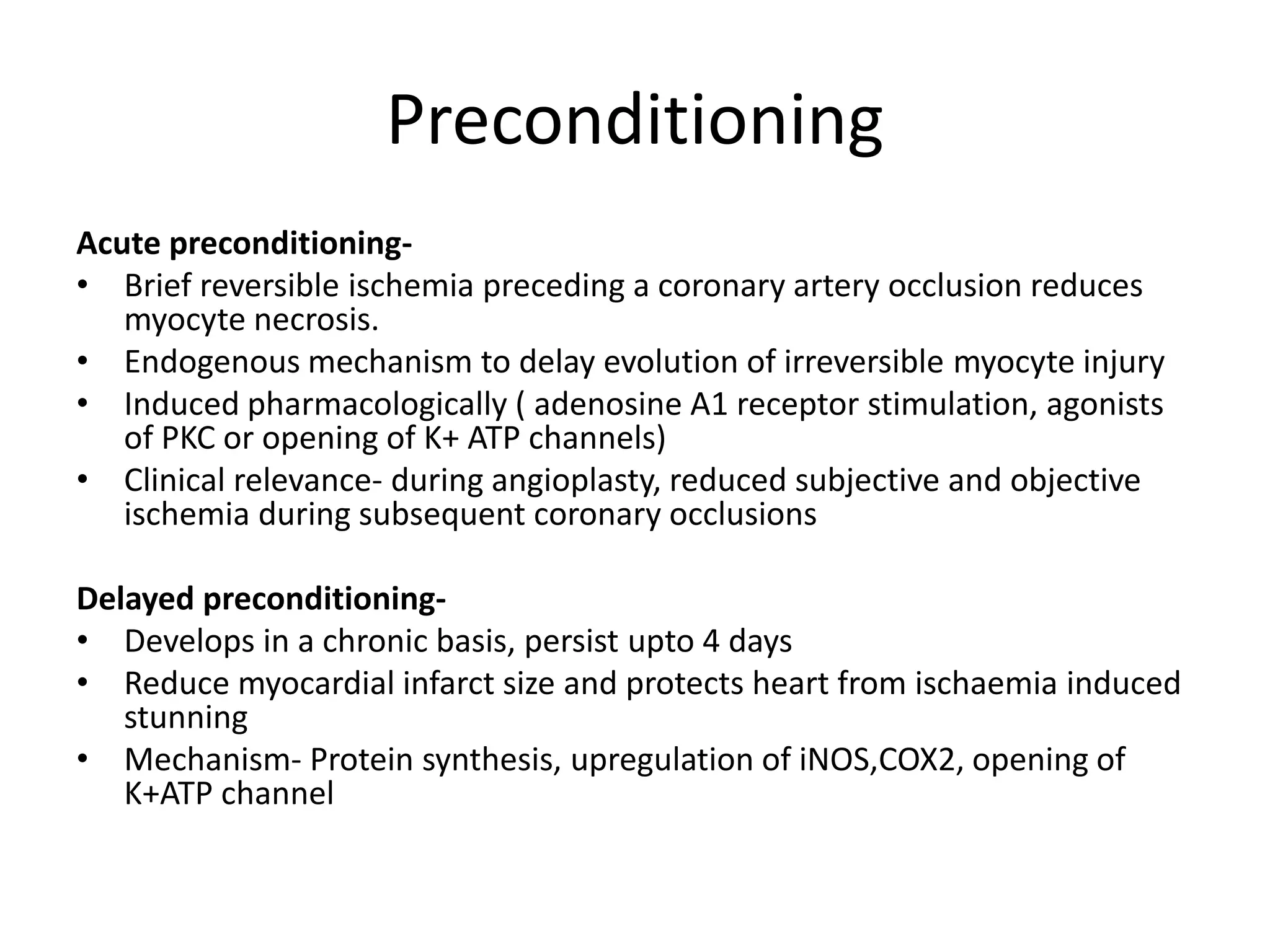
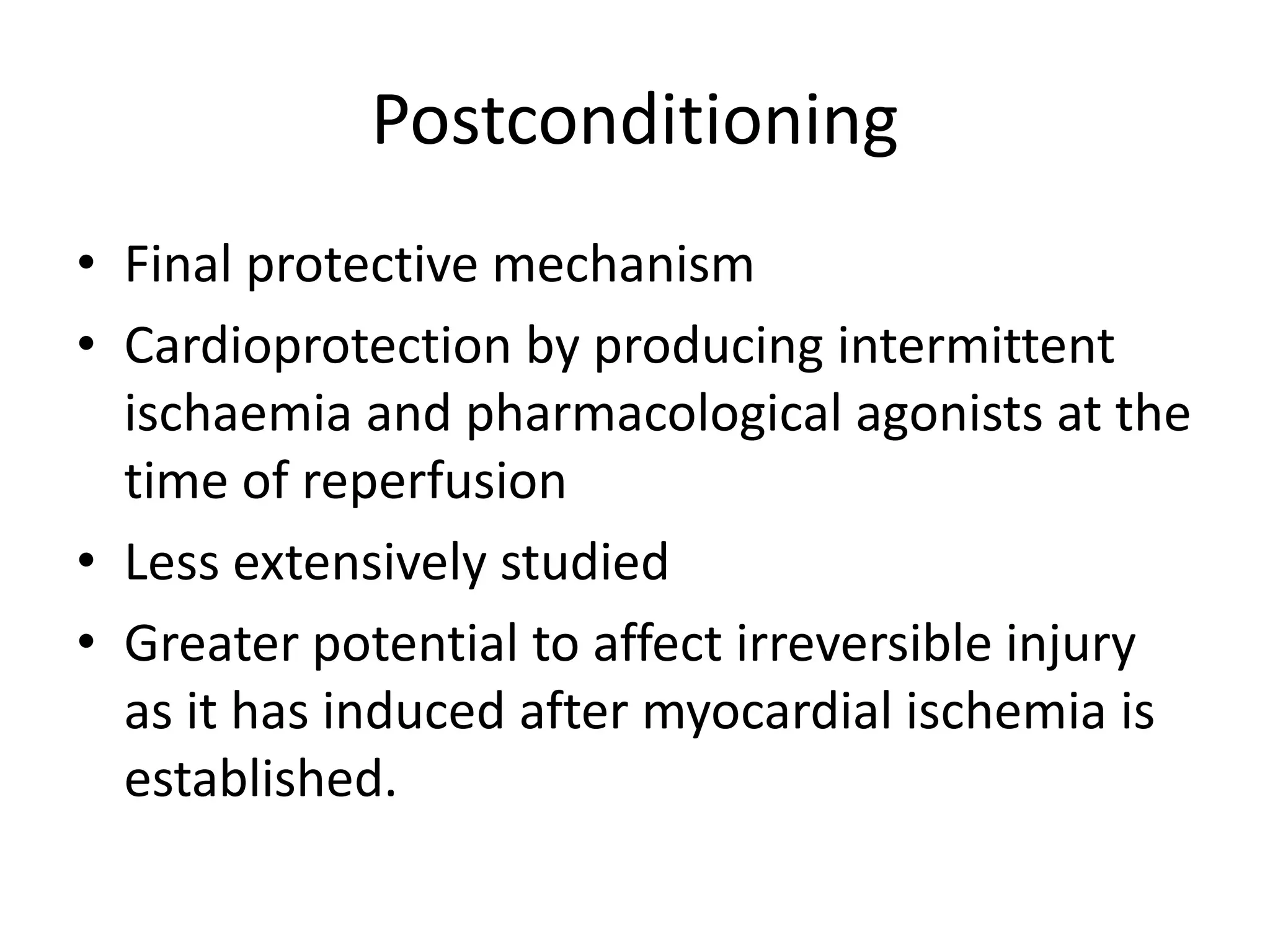
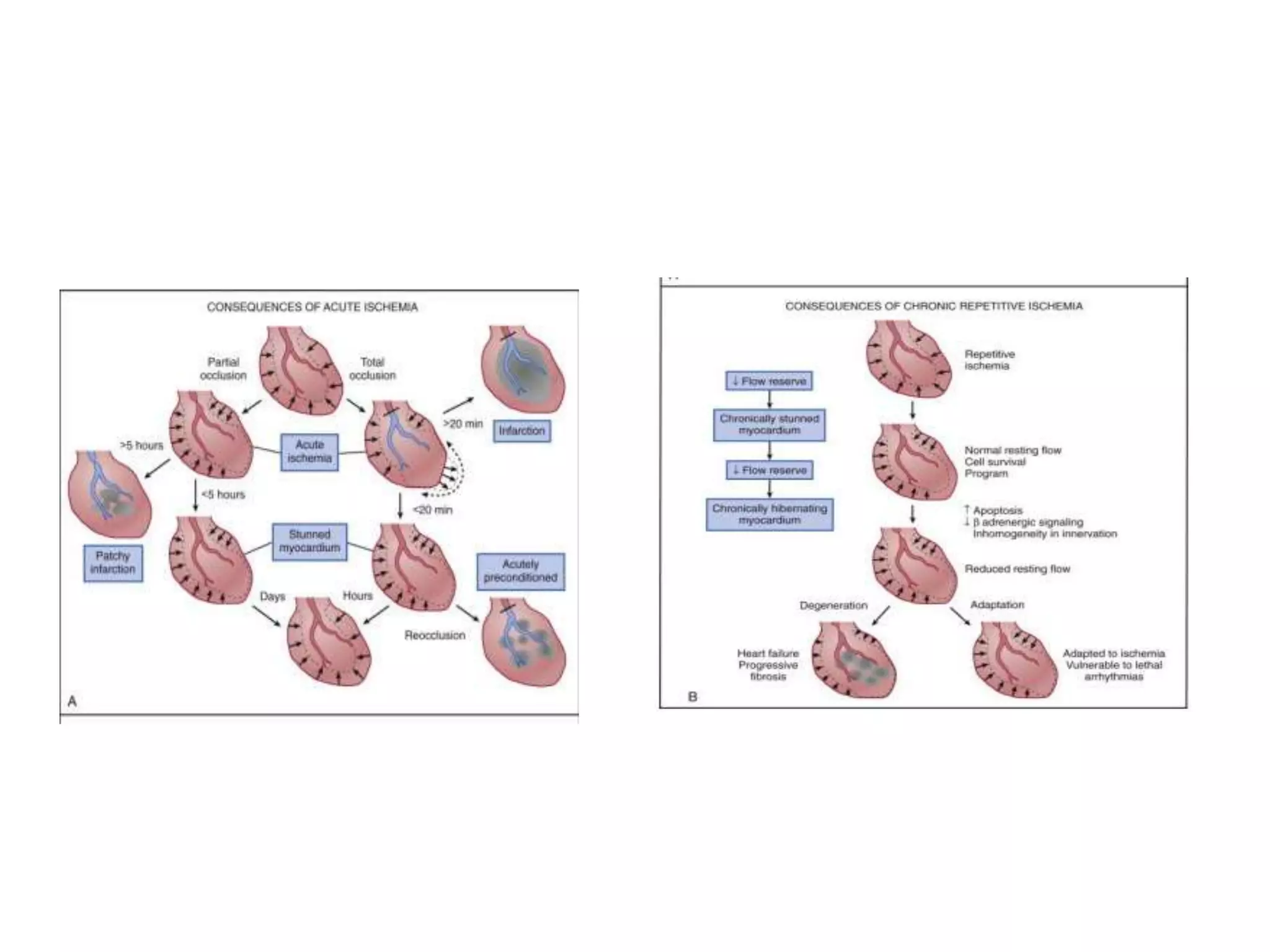
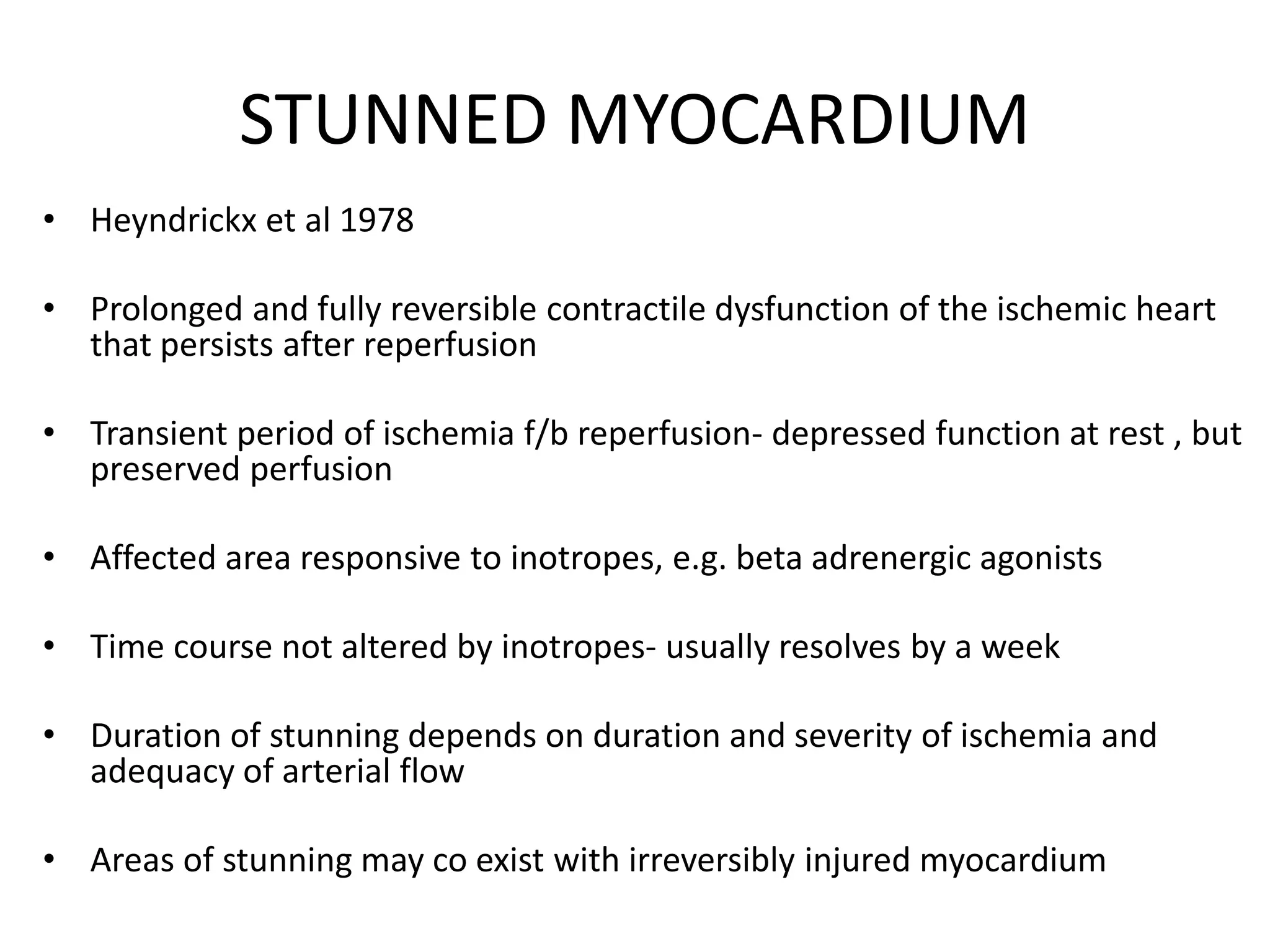
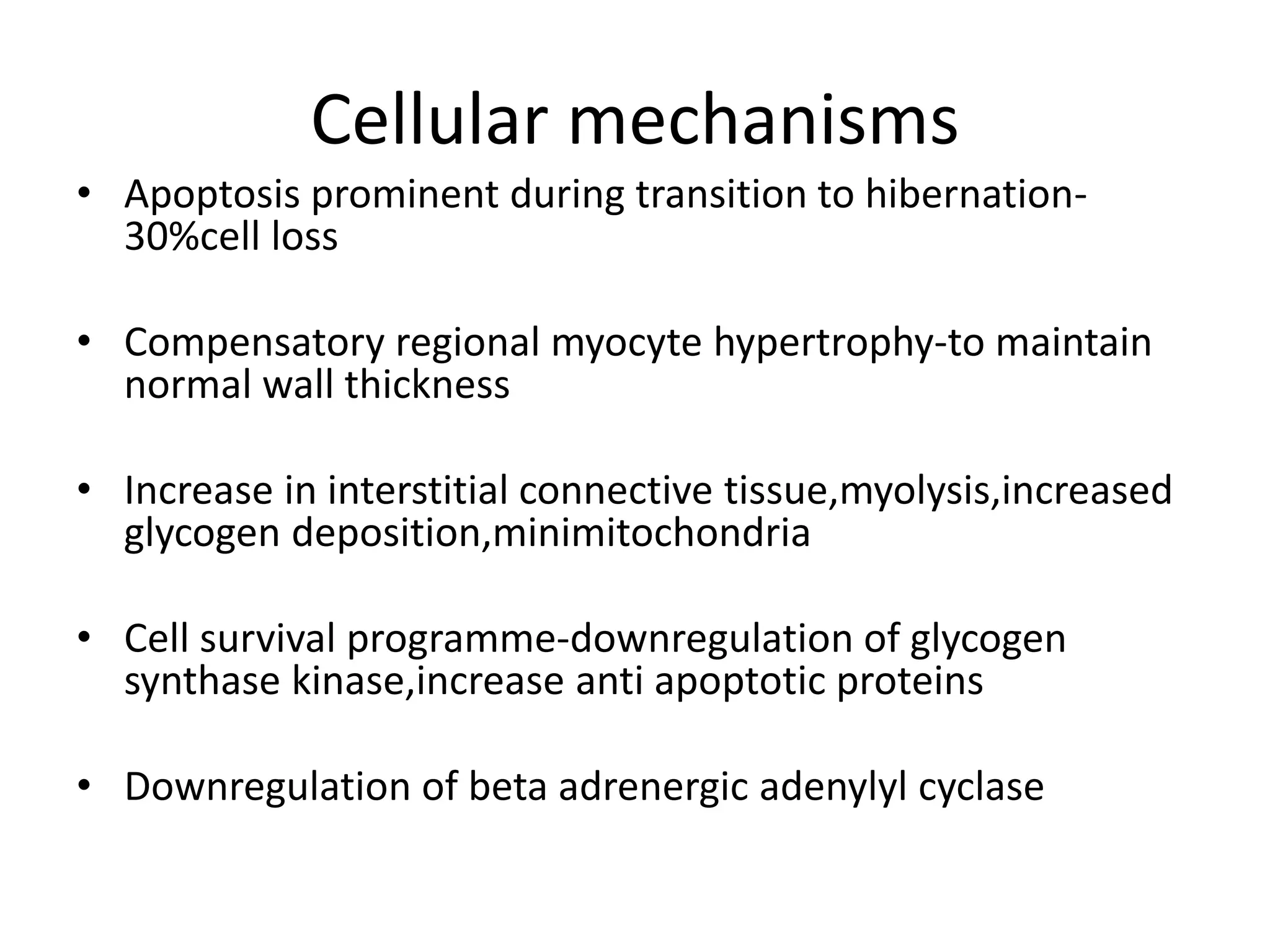
- Myocardial viability refers to dysfunctional tissue with limited scarring that has potential for functional recovery after revascularization through mechanisms like stunned myocardium and hibernation.
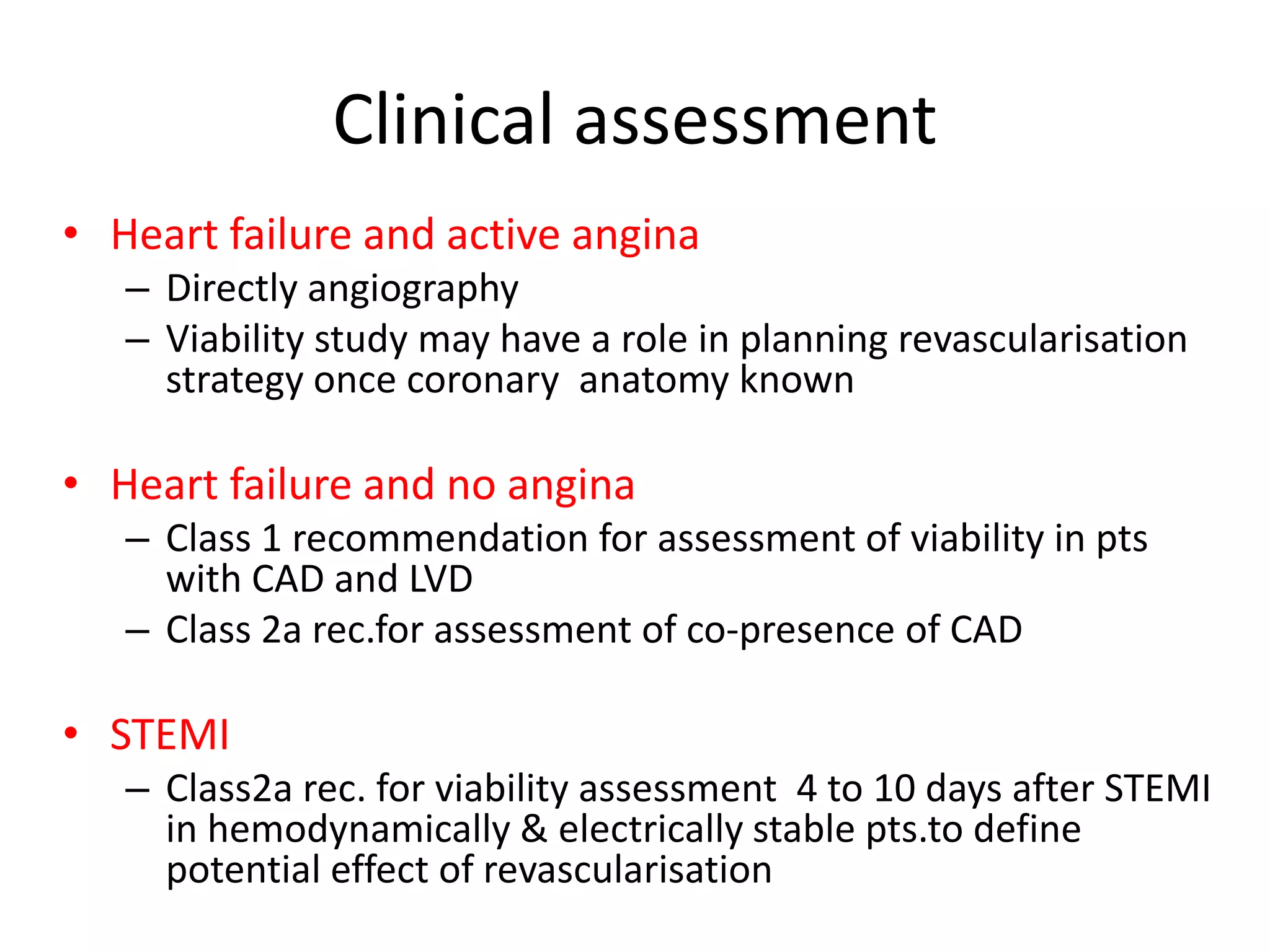
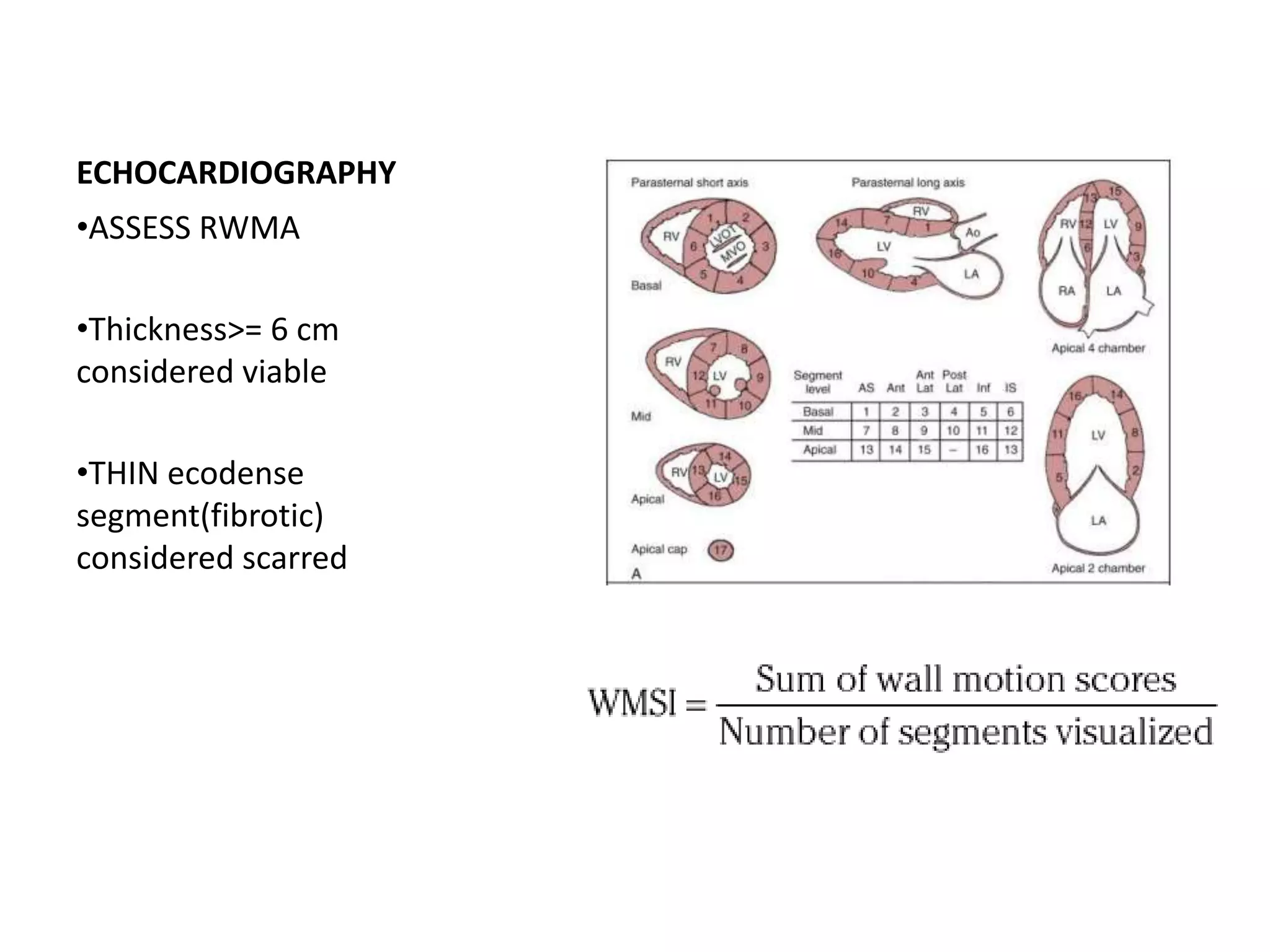
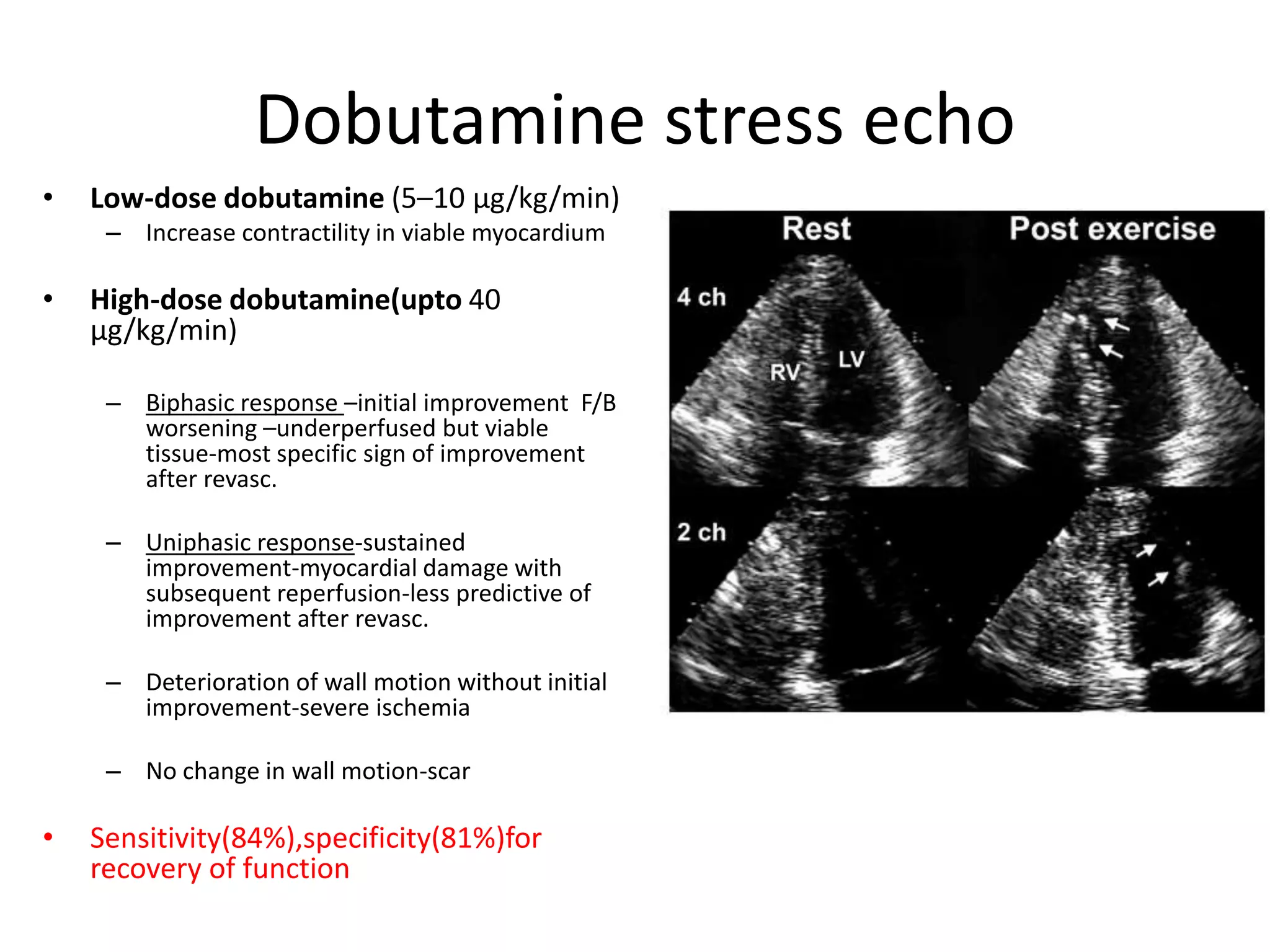
- Various techniques can assess viability including cardiac imaging and evaluating improvement in function after revascularization. Viability assessment aids decisions about revascularization