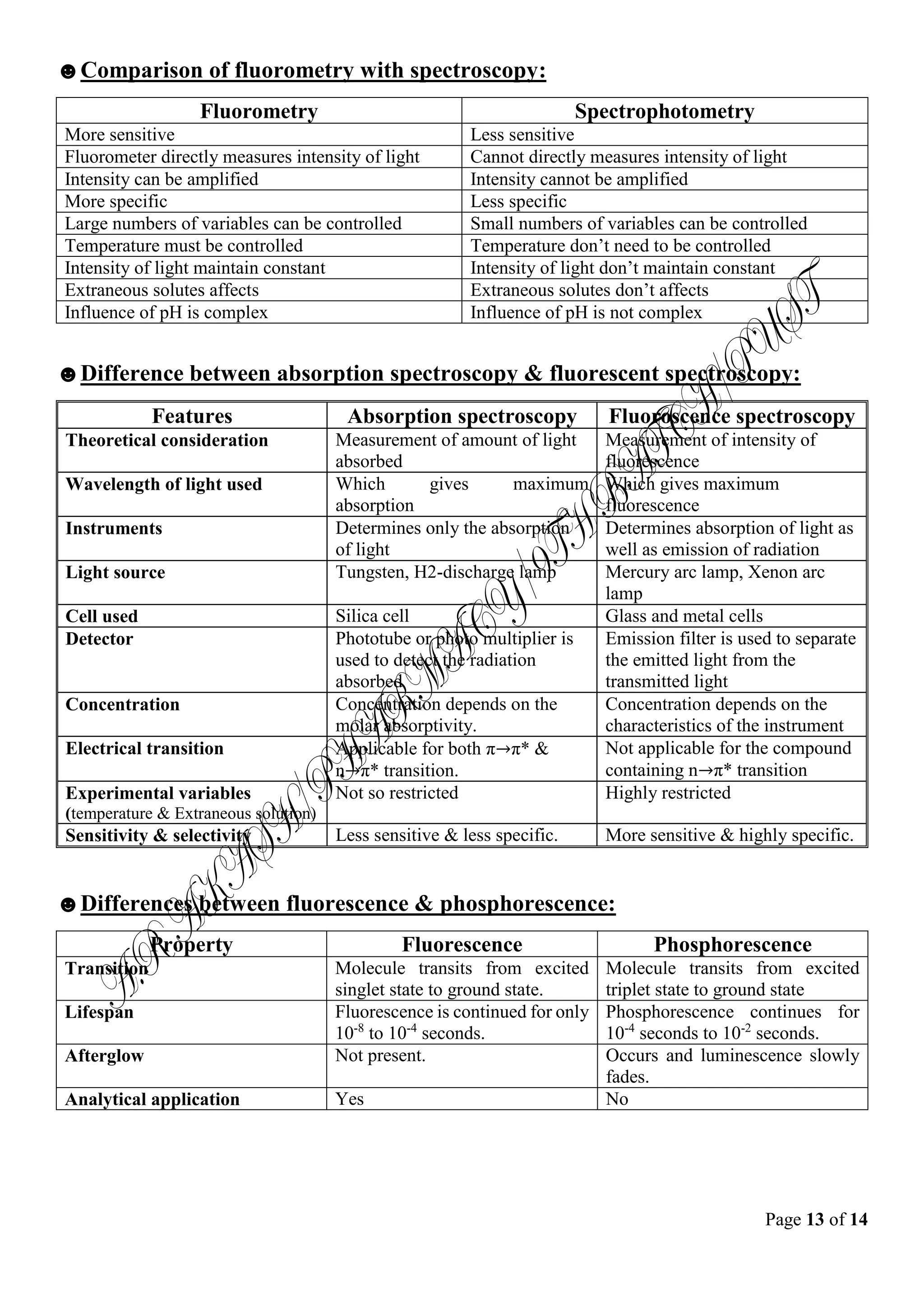
Fluorometry is an analytical technique that uses fluorescence to identify and characterize small amounts of substances. It involves exciting a sample with ultraviolet or visible light, which causes certain molecules to absorb energy and reach an excited electronic state. The molecules then emit light of a longer wavelength as they fall back to the ground state, and the intensity and composition of this fluorescent light can be measured. Fluorometric methods have applications in pharmaceutical analysis to measure compounds like riboflavin, thiamine, and reserpine in drug formulations.

![Page 2 of 14
This results from two intersystem crossings, first from the singlet to the triplet, then from the triplet to the
singlet.
Fluorescence is a kind of a luminescence, which is the emission of photons from electronically excited states.
Fluorescence occurs when the electron is transferred from a lower energy state into an "excited" higher energy
state. The electron will remain in this state for 10⁻⁸ sec. then the electron returns to the lower energy state and
it releases the energy in form of fluorescence. In ultraviolet absorption spectroscopy when molecule absorbs
UV radiation at one wavelength and it’s immediately re-emission, usually in a longer wavelength. Some
molecules fluoresce naturally and others can be modified to make fluorescent compounds.
The phenomenon of radiation emission during
transition from the lowest vibrational energy level of
the excited singlet state to the ground state is called
fluorescence.
☻Fluorometry: It is measurement of
fluorescence intensity at a particular wavelength
with the help of a filter fluorimeter or a
spectrofluorimete. It’s measured by a fluorometer or
fluorimeter.
☻Phosphorescence: Phosphorescence is defined as the emission of radiation by a chemical species during
its transition from the excited triplet state to the singlet ground state. The triplet state of a molecule has a lower
energy than its associated singlet state so that transitions back to the ground state are accompanied with the
emission of light of lower energy than from the singlet state. Therefore, we would typically expect
phosphorescence to occur at longer wavelengths than fluorescence. Phosphorescence is often characterized
by an afterglow because of the long life of the triplet state, 10-4
-10 seconds.
An important feature of phosphorescence is
afterglow. Light is emitted from phosphorescent
molecules after radiation energy source is removed.
This is because the luminescence continues for 10-4
seconds to 10 seconds as the triplet state has greater
longevity. In phosphorescence, similar to the
fluorescence, vibrational relaxation must occur.
So, Phosphorescence may be defined as emission of
radiation resulting from transition of molecule from
excited triplet state to ground state.
☻Singlet state (SS): Singlet state
is the state in which all of the electrons
are paired and in each pair the two
electrons spin about their own axis in
opposite directions.
At the ground state, the molecular orbitals are occupied by two electrons. The spins of the two
electrons in the same orbital must be antiparallel.This implies that the total spin, S, of the molecule
in the ground state is zero [½ + (-½)]. This energy state is called “singlet state” and is labeled as S0.](https://image.slidesharecdn.com/fluorometry-pdf-200714085457/75/Fluorometry-2-2048.jpg)