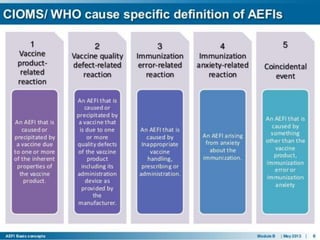
Pharmacovigilance is the science of monitoring the effects of pharmaceutical products after they have been licensed for use, especially in order to identify and evaluate previously unreported adverse reactions. It aims to improve patient care and safety, public health, benefit-risk assessment of medicines, and understanding of pharmacovigilance. Similarly, materiovigilance monitors medical devices and hemovigilance monitors the blood transfusion system to protect patient safety. Adverse events following immunization (AEFI) surveillance also monitors vaccine safety. Together these systems aim to enhance safety across all medical treatments.