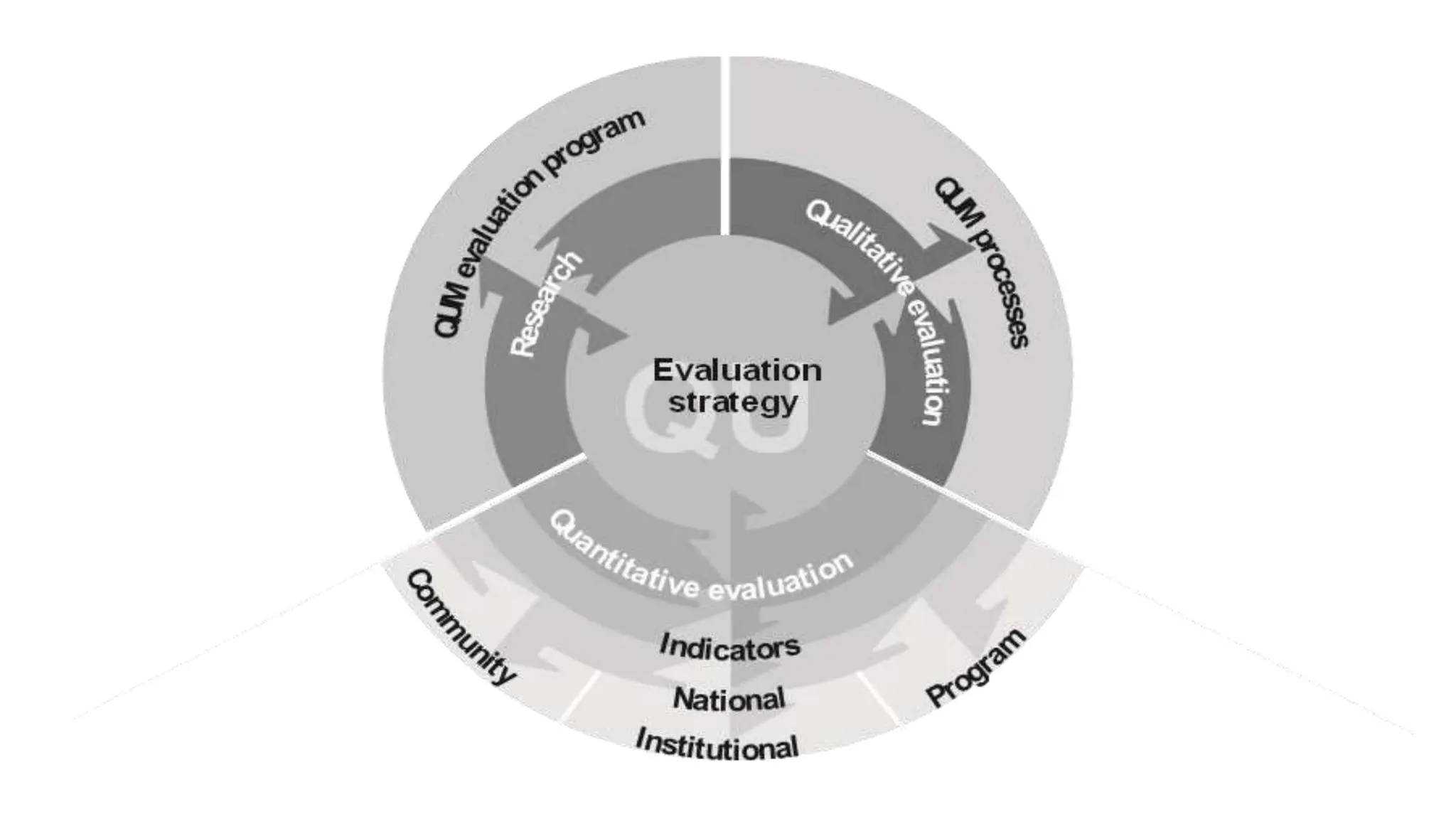
The document discusses Quality Use of Medicines (QUM) in Australia. It defines QUM as selecting management options wisely, choosing suitable medicines if needed and using medicines safely and effectively. The key principles of QUM are the primacy of consumers, partnership, consultative and collaborative activities, supporting existing activity, and systems-based approaches. Key partners in QUM include consumers, healthcare providers, educators, facilities, industries, media, funders and governments. The building blocks that support QUM are policy development, coordination, information provision, education and training, services, and research. Evaluation of QUM occurs at the community, institutional and national levels.