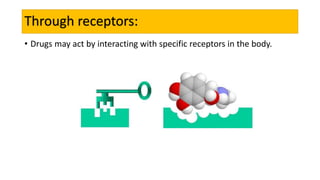
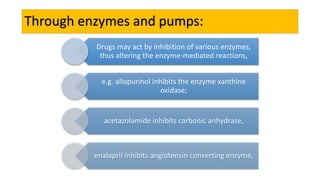
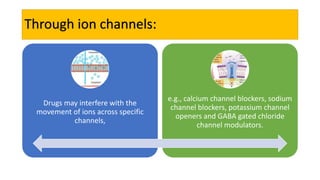
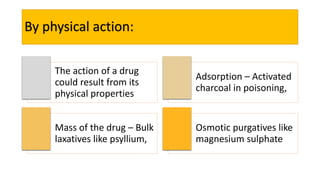
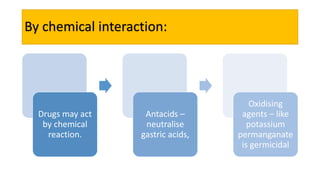
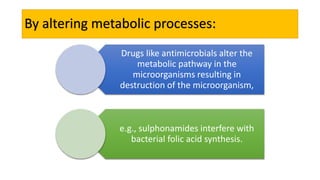
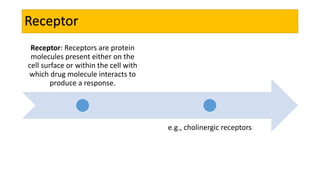
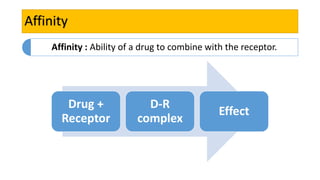
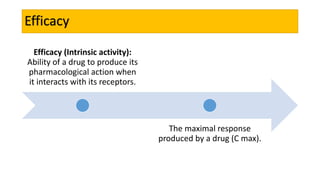
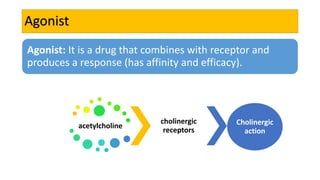
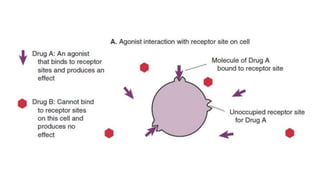
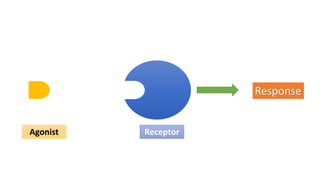
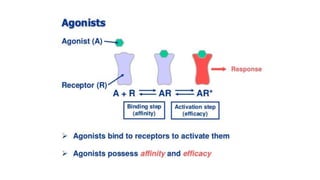
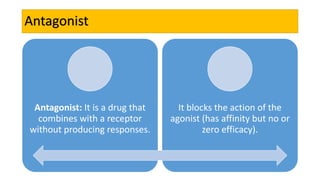
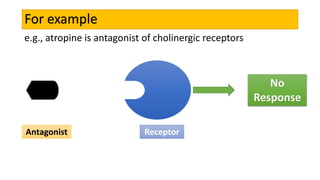
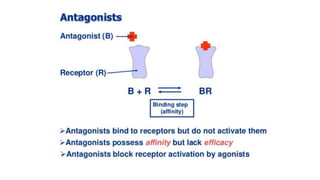
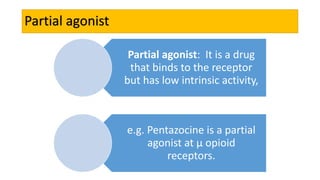
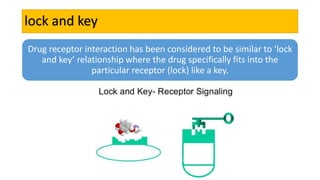
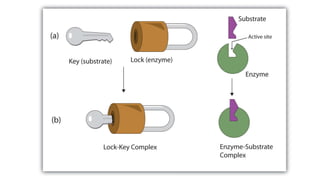
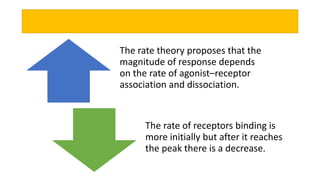
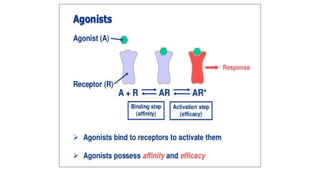
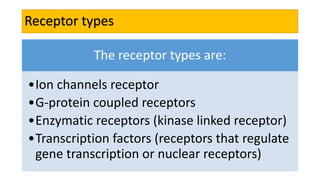
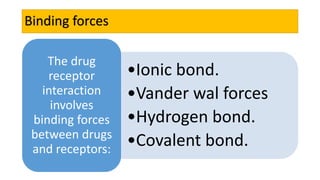
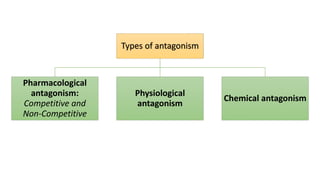
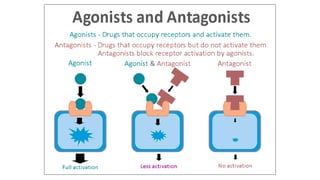
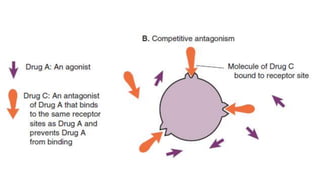
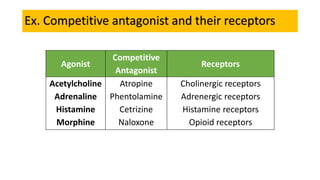
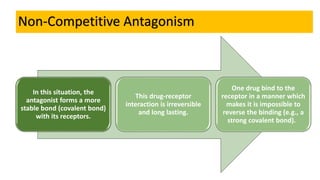
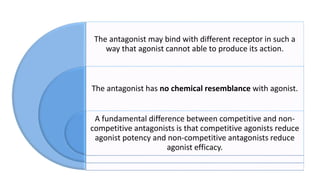
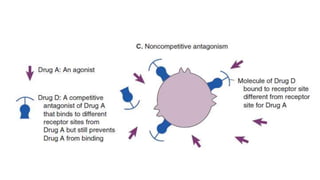
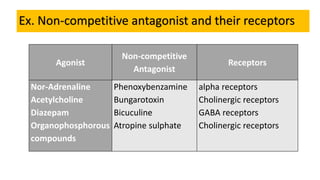
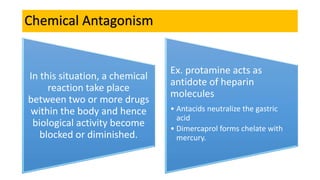
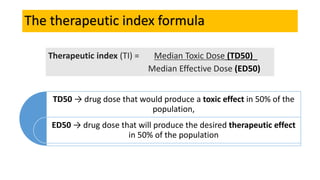
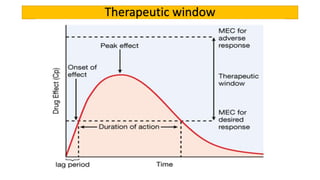
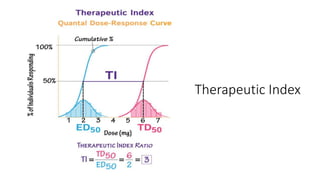
La farmacodinamia estudia los efectos bioquímicos y fisiológicos de los medicamentos y sus mecanismos de acción, interactuando de manera específica con los receptores proteicos del cuerpo. Las drogas pueden aumentar o disminuir la actividad celular, además de tener efectos de irritación, acción química o acción citotóxica, dependiendo del tipo de receptor y la interacción con otros medicamentos. Se destaca la importancia del índice terapéutico para medir la seguridad de un fármaco, diferenciando entre dosis efectivas y tóxicas.