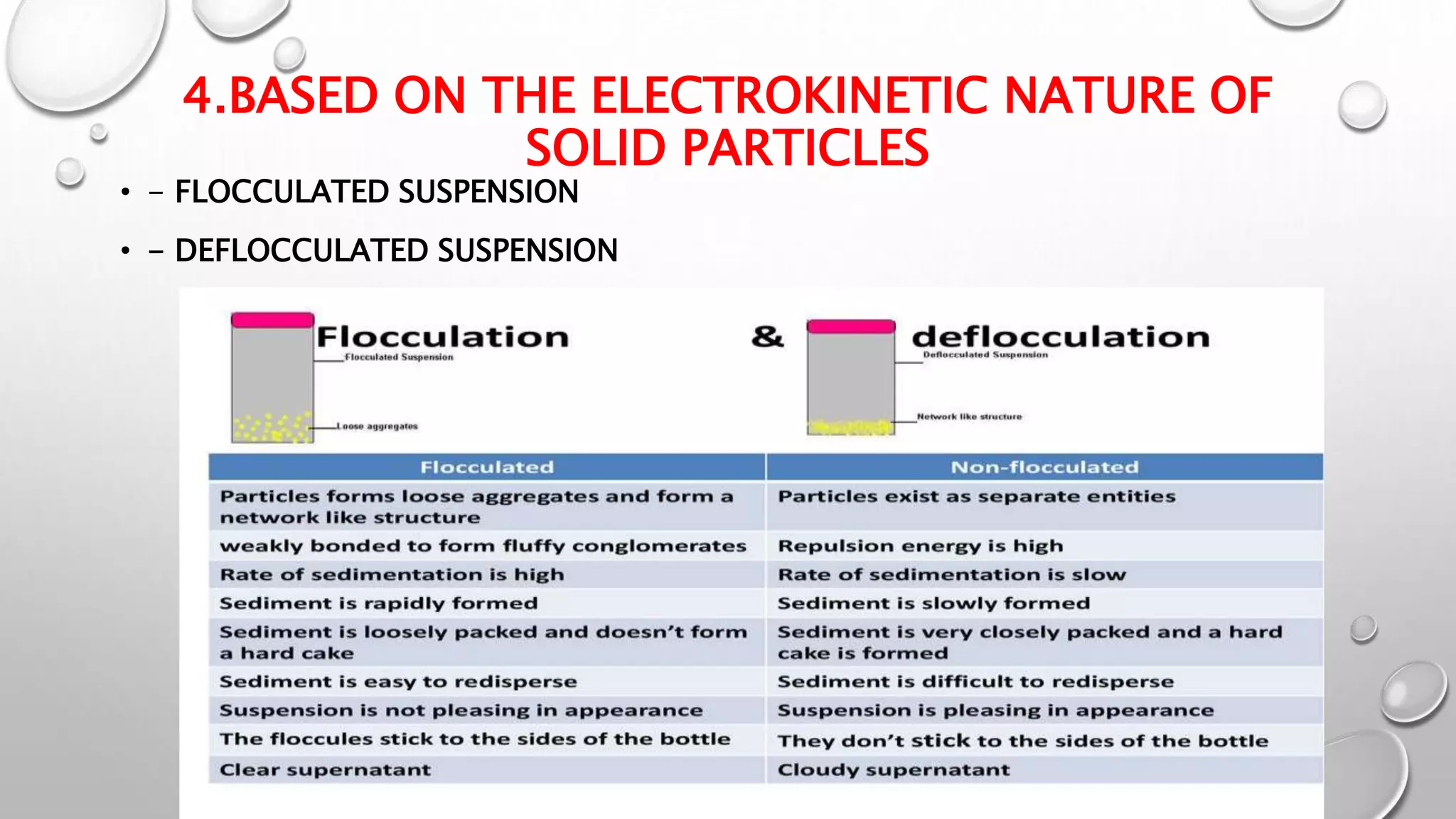
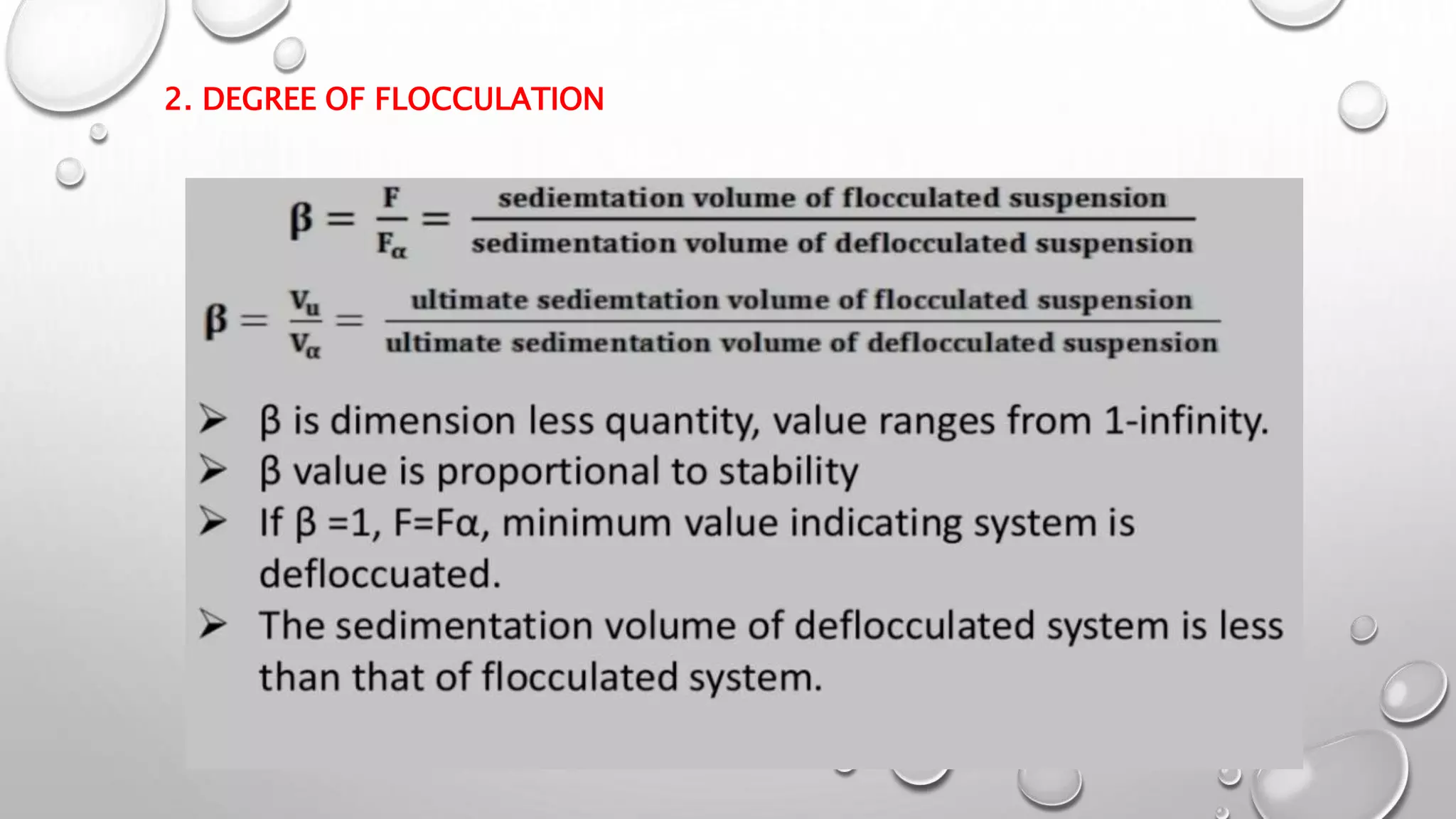
The document discusses suspensions as biphasic liquid dosage forms, detailing their definitions, advantages such as enhanced bioavailability and taste masking, and disadvantages including sedimentation and formulation challenges. It covers ideal properties for good suspensions, classification based on administration routes, solid particle proportions, sizes, and electrokinetic nature. Additionally, it outlines key components and agents used in suspension formulations along with methods for evaluating their stability and performance.