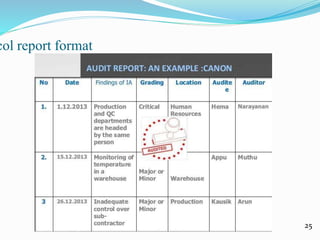
This document discusses quality auditing in the pharmaceutical industry. It defines quality auditing and explains that it involves systematically examining quality systems to determine compliance. The document outlines different types of audits including internal, external, regulatory, product, process, and system audits. It discusses planning, conducting, and reporting on audits. The key objectives of audits are to ensure quality, assess effectiveness of quality assurance systems, and permit timely correction of any issues. Audits help build confidence in quality management practices and identify areas for improvement.