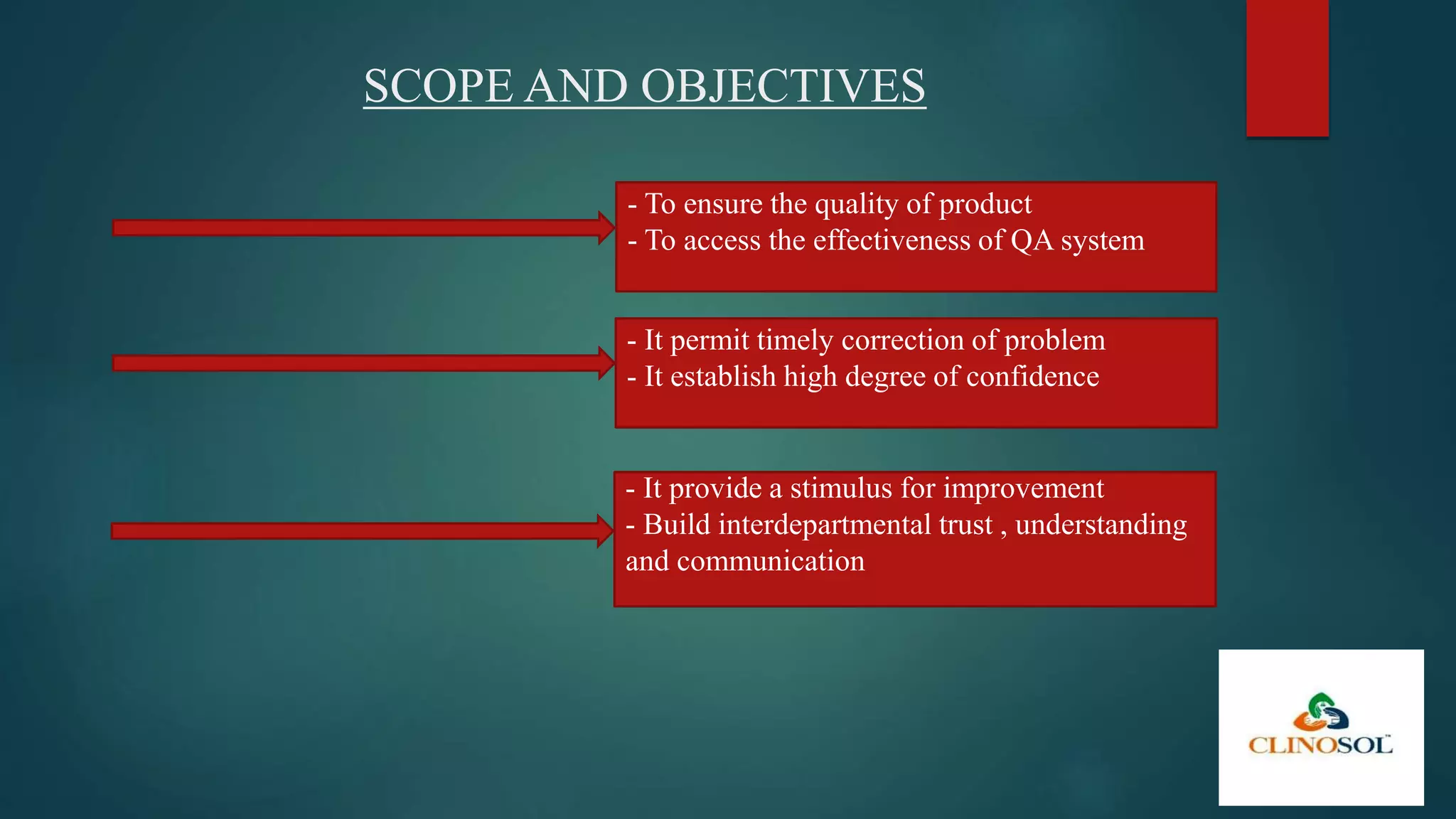
A quality audit is an independent examination to assess compliance with quality standards and effectiveness in achieving objectives, typically conducted at defined intervals. It serves to ensure product quality, improve QA systems, and build interdepartmental trust. Audits can be internal or external, are vital for regulatory compliance, and help identify potential problems and necessary improvements in manufacturing processes.











![Who performs an Audit?
[A] Imposed upon
manufacturer or
supplier
[B] Performed by
manufacturer
Regulatory
Customer or
Potential customer
Third party
Internal{Self-
Inspection}
External
• Overall
• Departmental
• Product-based
• System-oriented
• Supplier
• Contract manufacturer
• Packager](https://image.slidesharecdn.com/14-230523094458-4fa05be0/75/Quality-Audit-13-2048.jpg)







