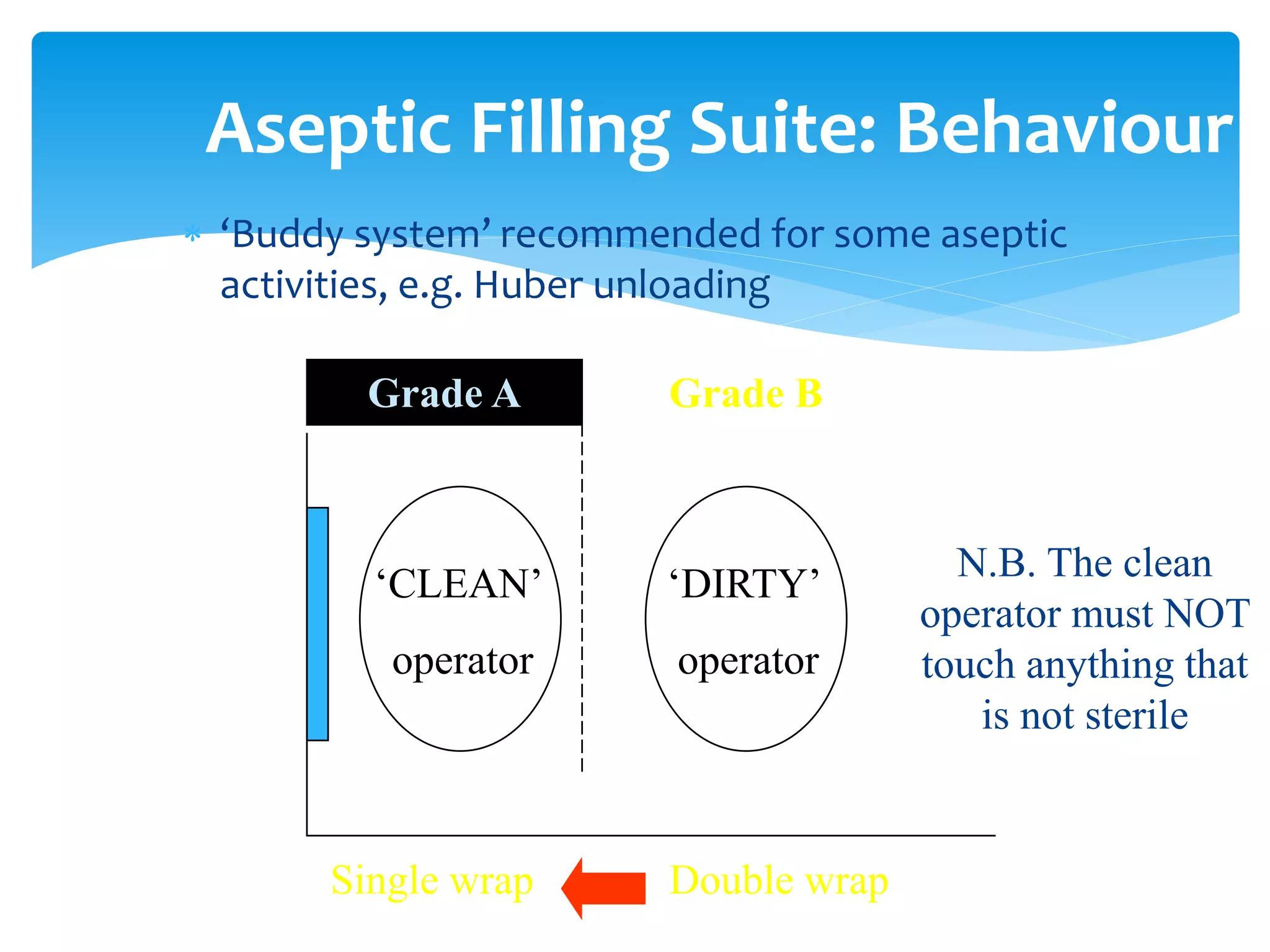
This document outlines standards of behavior and practices for cleanrooms and cleanzones. It defines key terms like cleanroom, cleanzone, critical activity, and aseptic processing. The document provides guidelines for behaviors in all cleanrooms and cleanzones, including proper gowning, minimizing contamination, and maintaining orderly work areas. It also outlines additional requirements for aseptic filling suites, such as proper glove sanitization procedures and using aseptic technique. The document states that compliance with these standards will be assessed through microbiological audits and is necessary to meet regulatory expectations for sterile drug production.





![ Aseptic processing:
where the drug product, container, and closure
are subject to sterilisation processes separately,
as appropriate, and then brought together
‘Any manual or mechanical manipulation of the
sterilised drug, components, containers and
closures prior to, or during, aseptic assembly
poses a risk of contamination and thus
necessitates careful control’
Sterile Drug Products Produced by
Aseptic Processing [FDA]](https://image.slidesharecdn.com/cleanroomsopslides-150726173527-lva1-app6892/75/Cleanroom-sop-slides-7-2048.jpg)
![ ‘Poor cGMP conditions…can ultimately pose a
life threatening health risk to a patient’
‘Even successfully qualified systems can be
compromised by:
poor personnel activities
poor operational activities
poor maintenance activities’
Sterile Drug Products Produced by
Aseptic Processing [FDA]](https://image.slidesharecdn.com/cleanroomsopslides-150726173527-lva1-app6892/75/Cleanroom-sop-slides-8-2048.jpg)
![ ‘It is essential that operators involved in aseptic
manipulations adhere to the basic principles of aseptic
technique at all times…’
Appropriate training should include:
cleanroom behaviour
aseptic technique
microbiology
gowning
patient safety hazard posed by a non-sterile product
Personnel training should be updated regularly
Supervisors should routinely evaluate operators
Sterile Drug Products Produced by
Aseptic Processing [FDA]](https://image.slidesharecdn.com/cleanroomsopslides-150726173527-lva1-app6892/75/Cleanroom-sop-slides-9-2048.jpg)