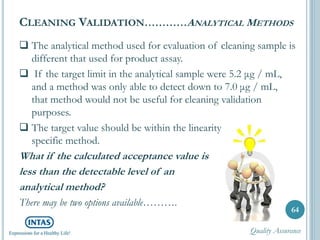
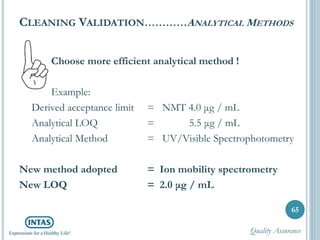
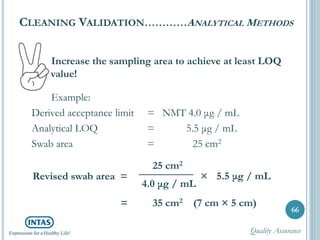
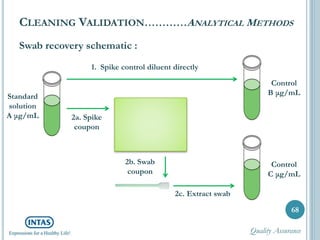
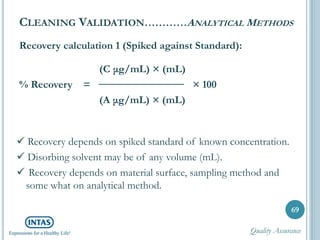
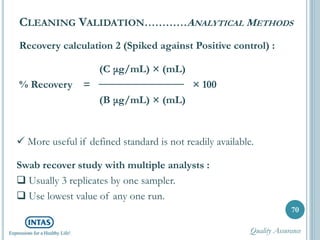
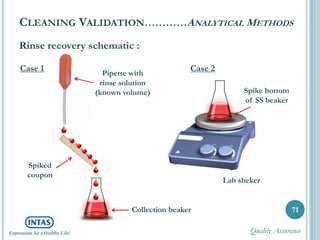
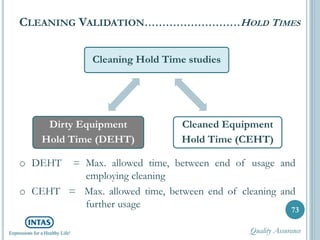
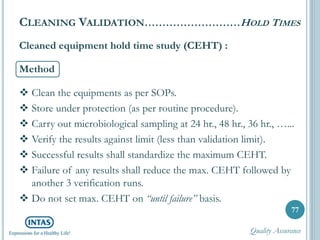
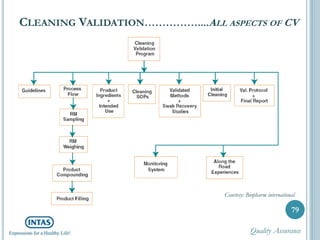
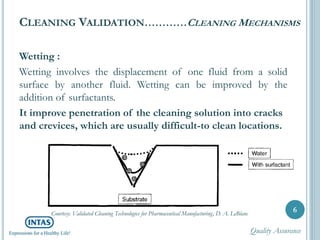
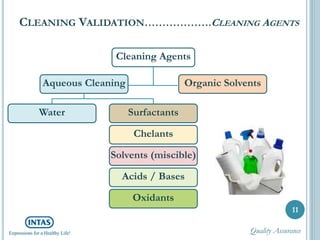
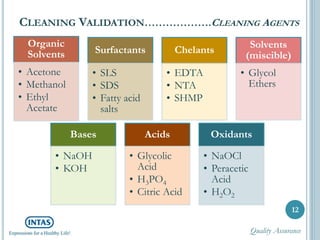
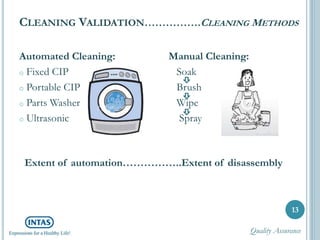
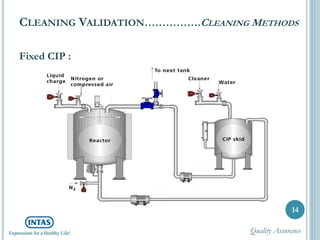
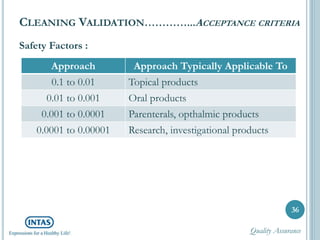
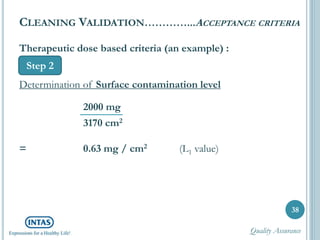
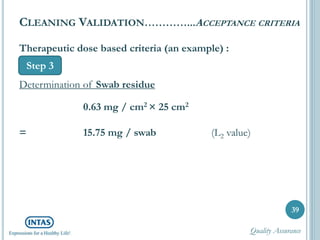
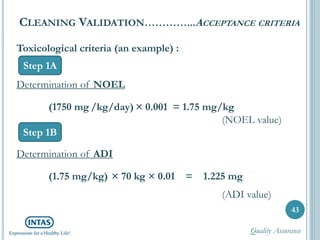
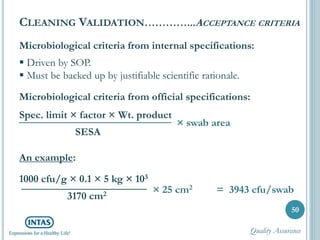
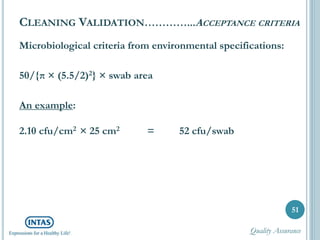
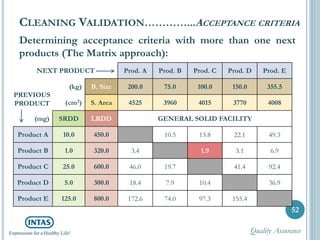
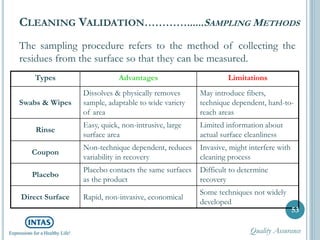
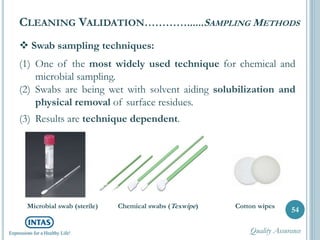
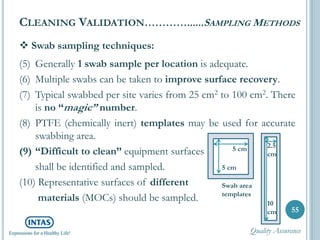
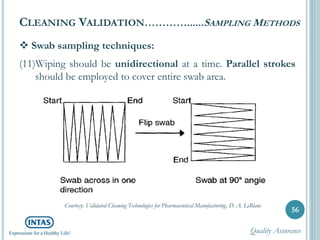
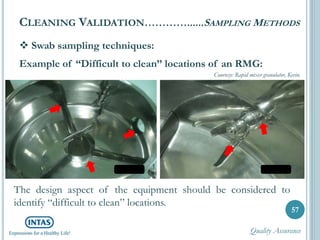
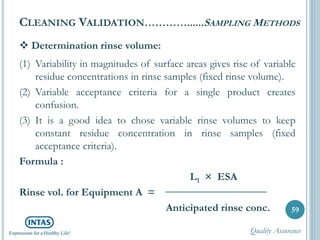
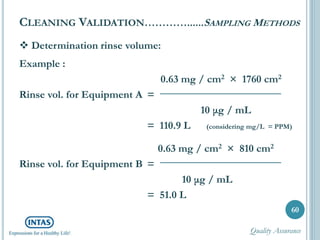
The document discusses cleaning validation, including definitions, purposes, mechanisms, agents, methods, parameters, groupings, worst case considerations, acceptance criteria, and more. Cleaning validation is defined as the process of removing contaminants from equipment and monitoring such that equipment can be safely reused. It aims to ensure product integrity, prevent cross contamination, and meet regulatory requirements.































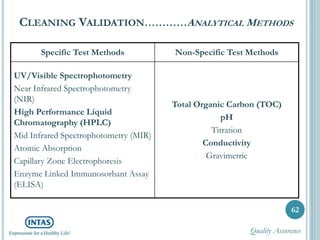
![The analytical methods used for testing cleaning samples
must be validated for [ICH Q2 (R1)]:
Limit of Detection (LOD)
Limit of Quantification (LOQ)
Specificity
Accuracy
Repeatability
Precision
Range
Linearity
Recovery
CLEANING VALIDATION…………ANALYTICAL METHODS
63
Quality Assurance](https://image.slidesharecdn.com/cleaningvalidationacompleteknowhow-140528034627-phpapp01/85/Cleaning-validation-a-complete-know-how-64-320.jpg)