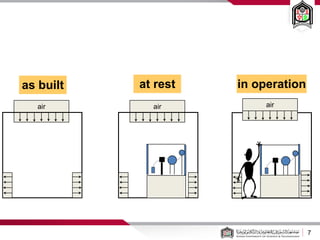
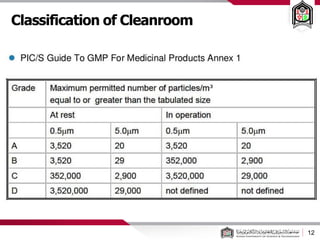
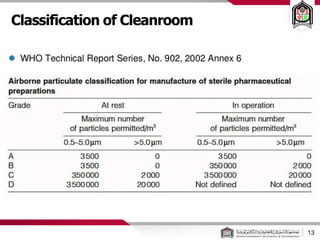
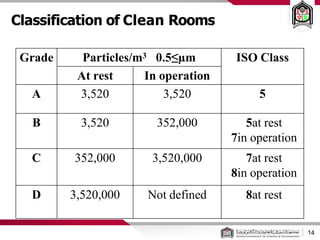
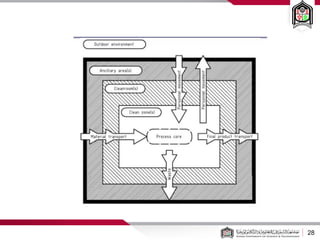
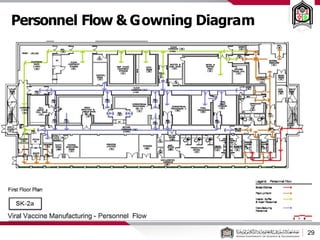
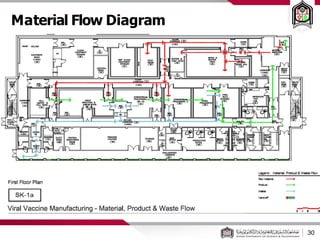
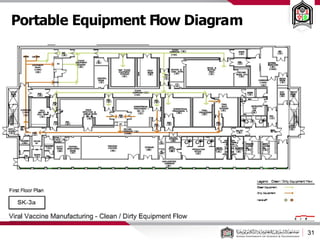
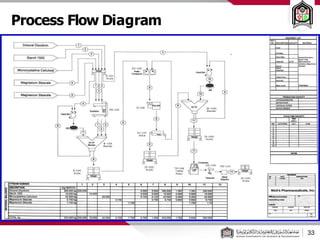
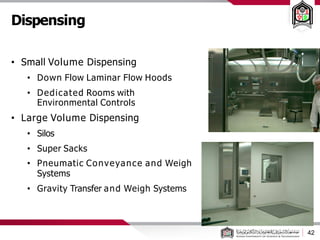
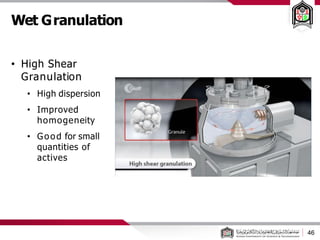
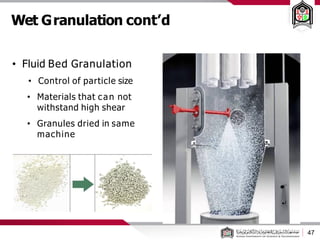
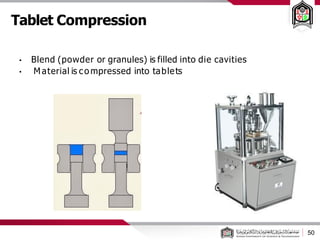
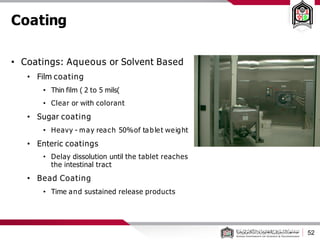
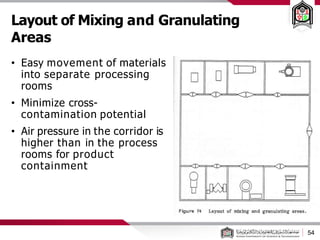
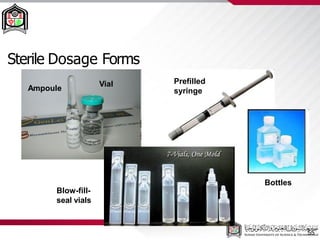
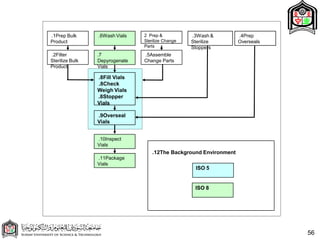
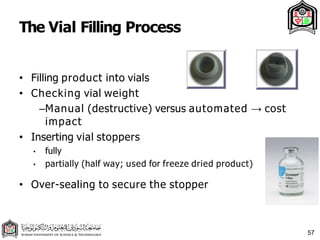
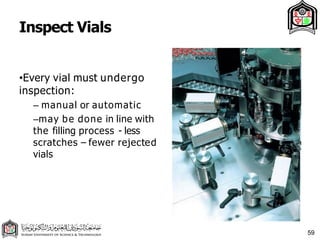
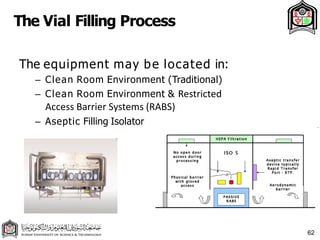
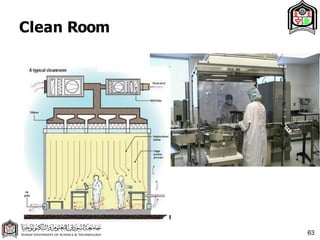
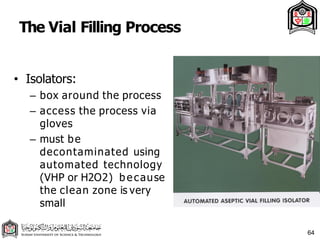
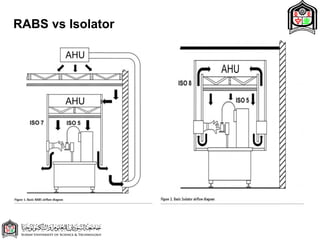
This document discusses cleanroom design and classification for pharmaceutical facilities. It begins by defining cleanrooms and their importance in industries like pharmaceuticals. It then explains cleanroom classification standards from ISO and PIC/S, which classify cleanrooms based on maximum allowable particle counts. The document outlines design considerations for cleanrooms, including airflow, materials, and features to enable cleaning. It emphasizes the importance of layouts and flow diagrams to design facilities that meet process, personnel, and material flow needs.