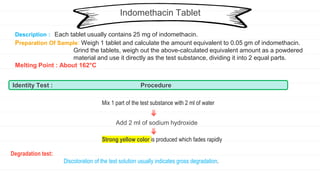
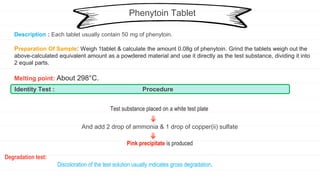
The document outlines procedures for quality control and standardization of various herbal and pharmaceutical dosage forms, detailing sample preparation and identity tests for each drug. It emphasizes the importance of maintaining quality throughout every stage of drug production, from material sourcing to distribution. Each section includes descriptions, preparation methods, and tests to confirm the identity and assess degradation of specific substances.