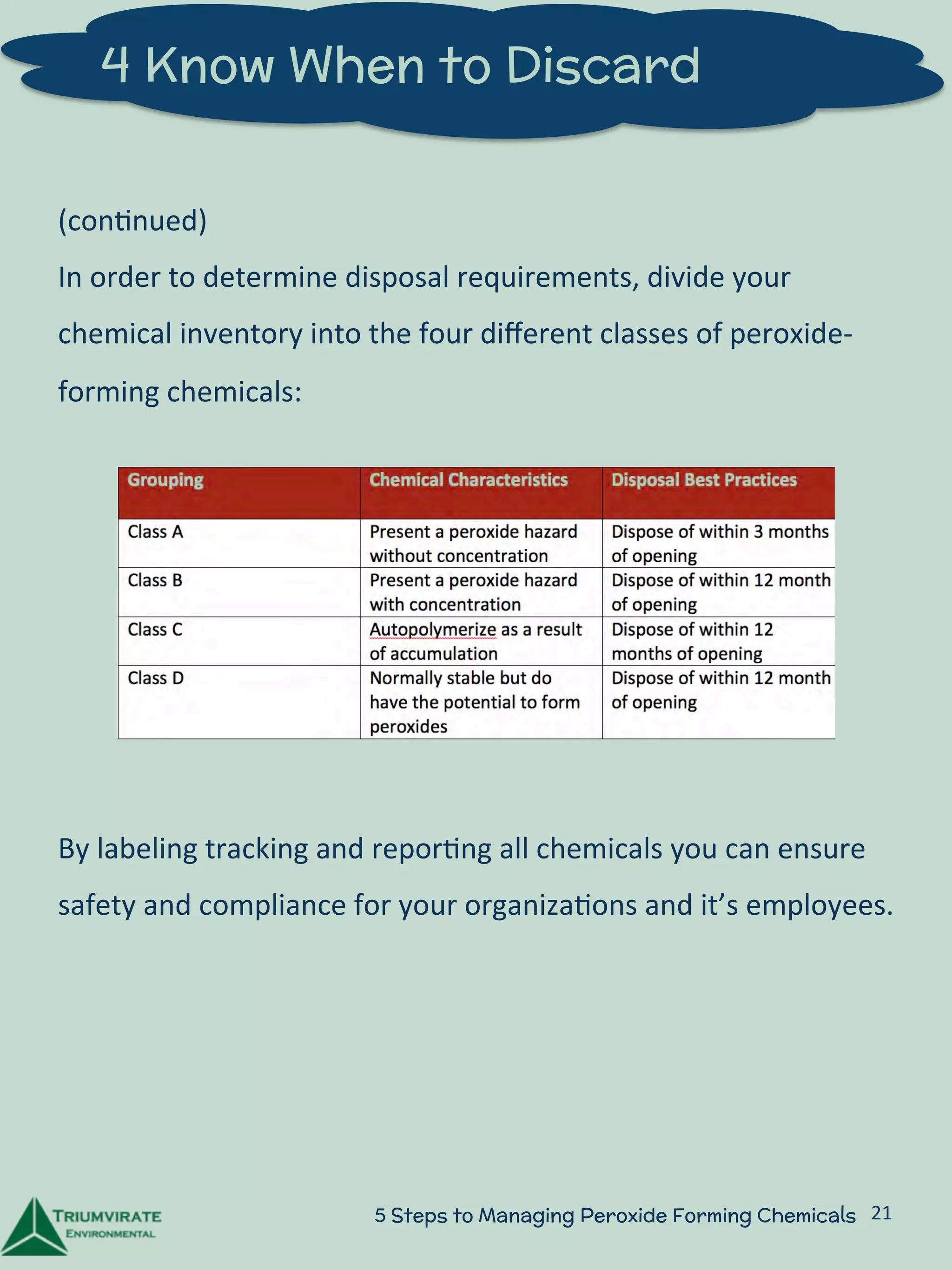
This document provides a 5-step guide to safely managing peroxide-forming chemicals in laboratories. Step 1 explains that peroxide formers are chemicals that can spontaneously form explosive peroxide compounds over time when exposed to factors like oxygen, light, or heat. Step 2 outlines protocols for proper storage, handling, and testing of peroxide formers. Step 3 recommends training researchers on identification and safety procedures for peroxide formers. Step 4 discusses determining appropriate disposal times based on chemical hazard classifications. Step 5 suggests developing long-term plans for continual education and chemical inventory management. The document aims to help laboratories avoid accidents from improperly managed peroxide-forming chemicals.