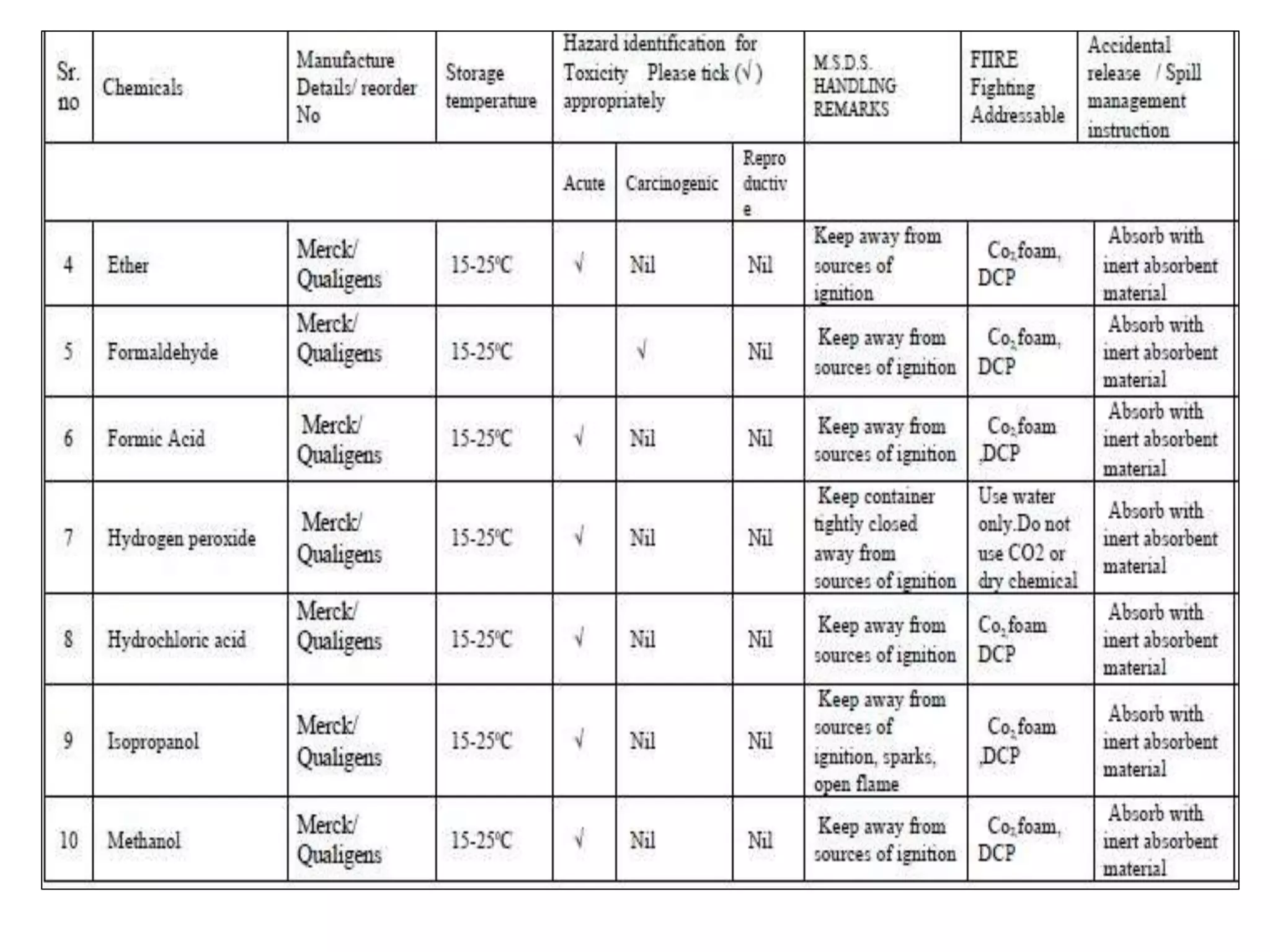
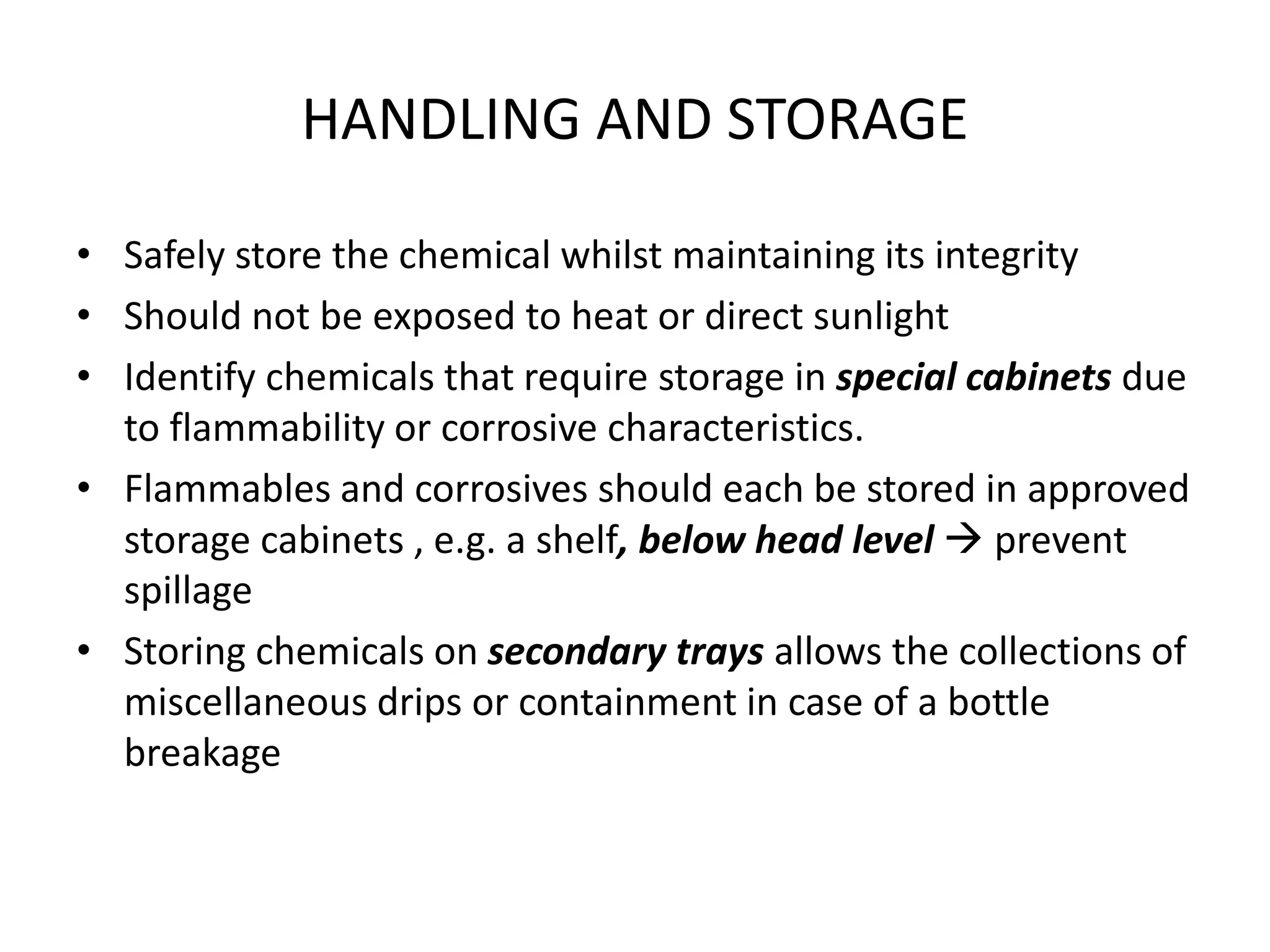
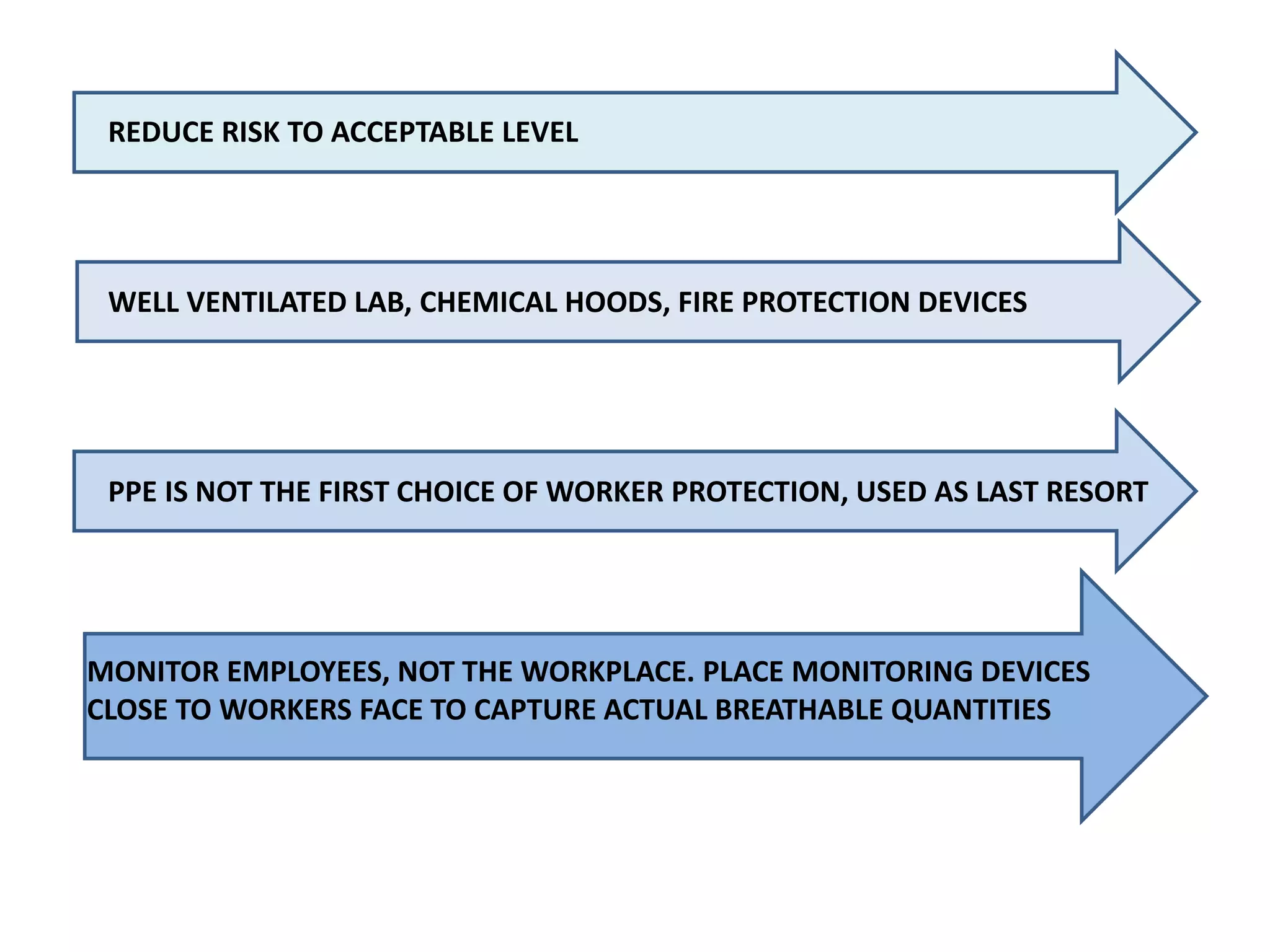
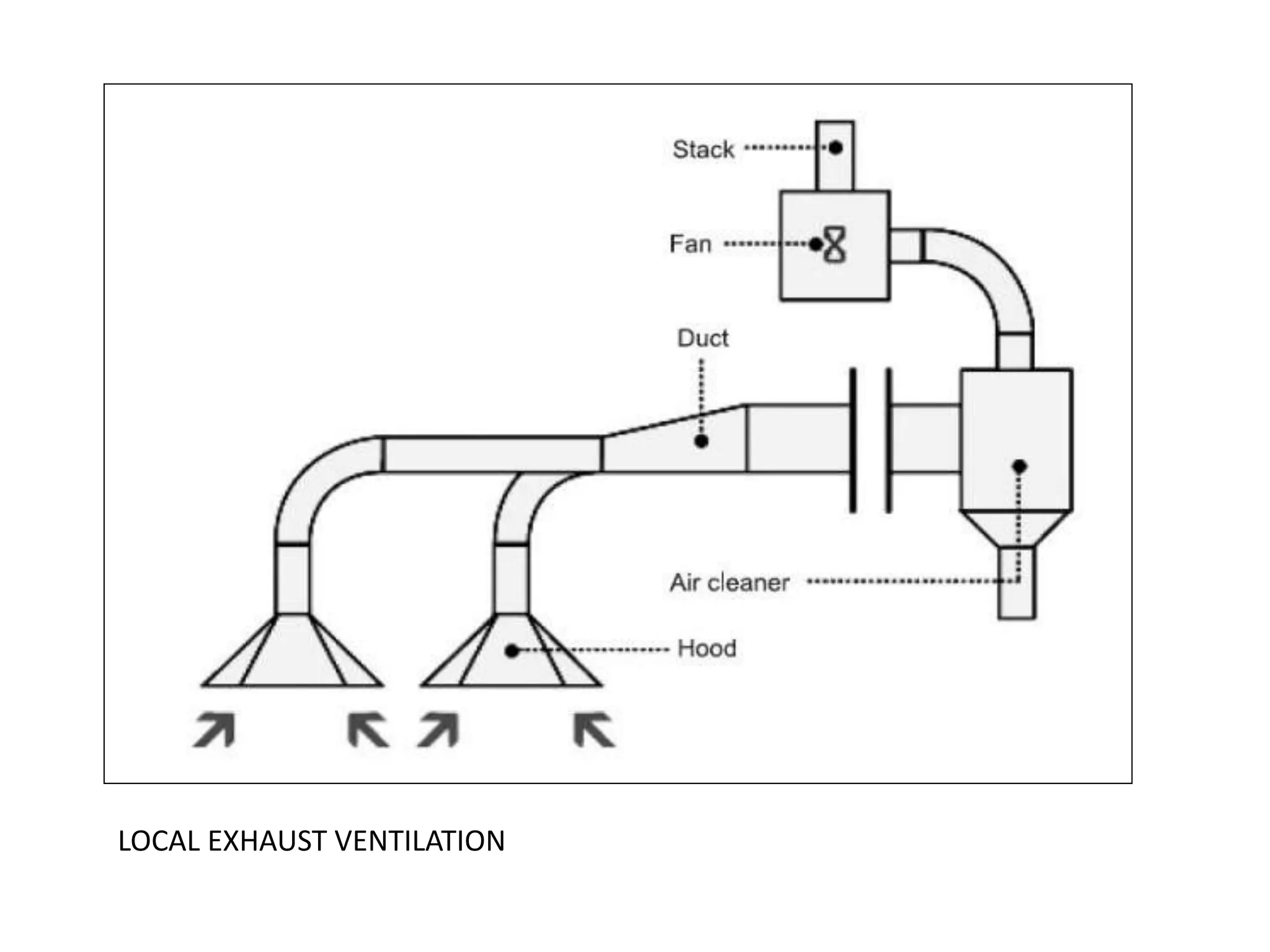
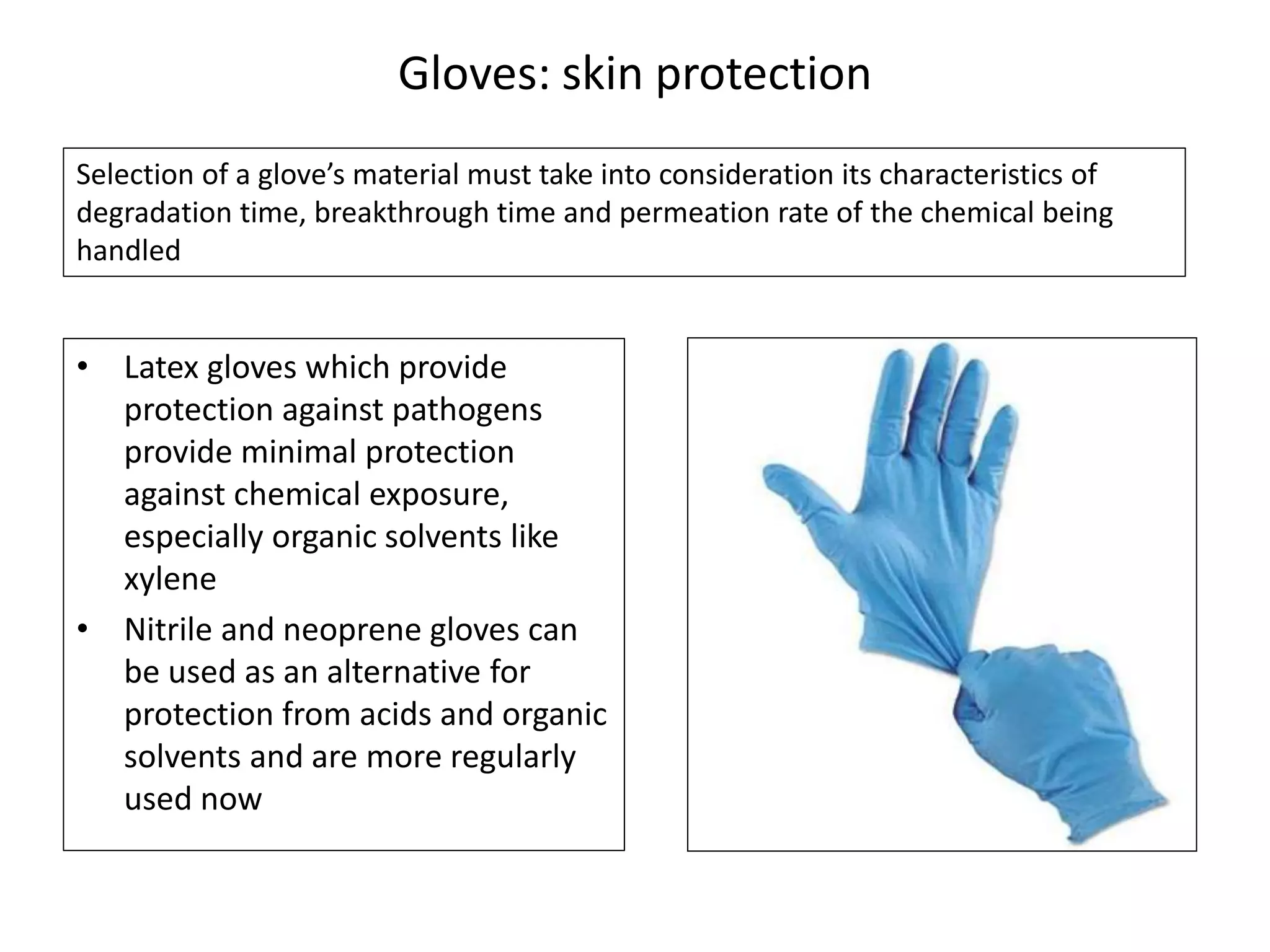
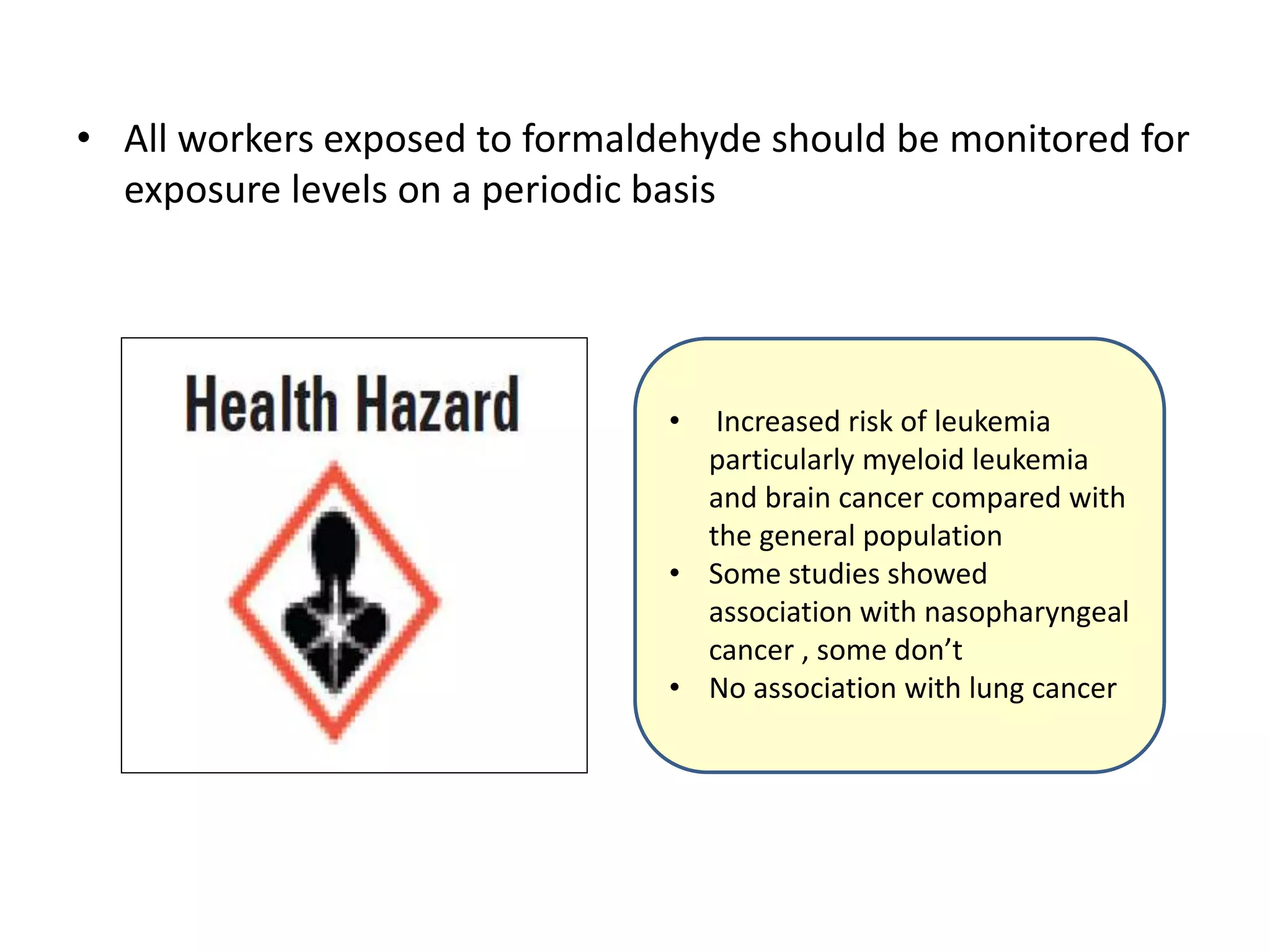
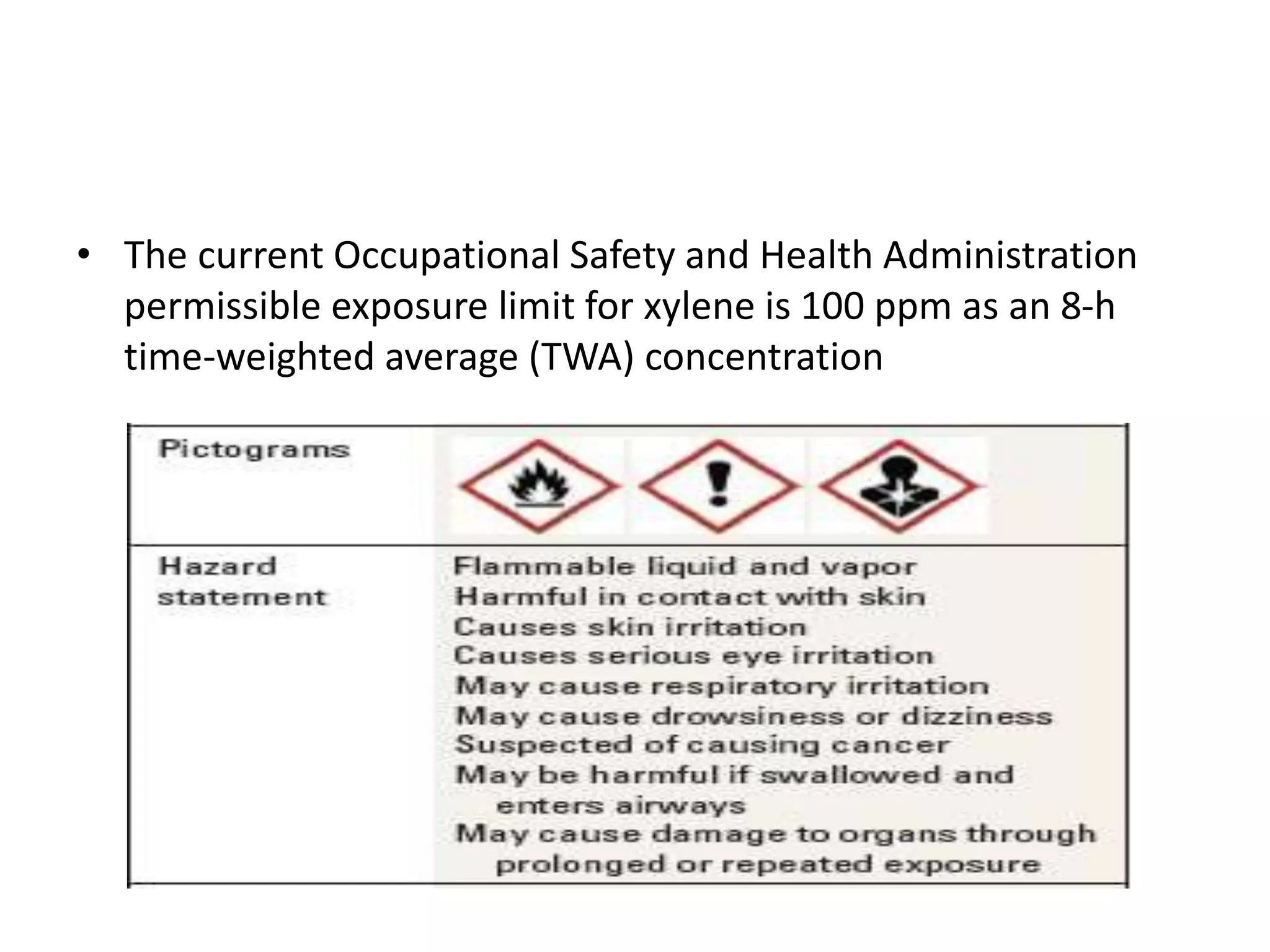
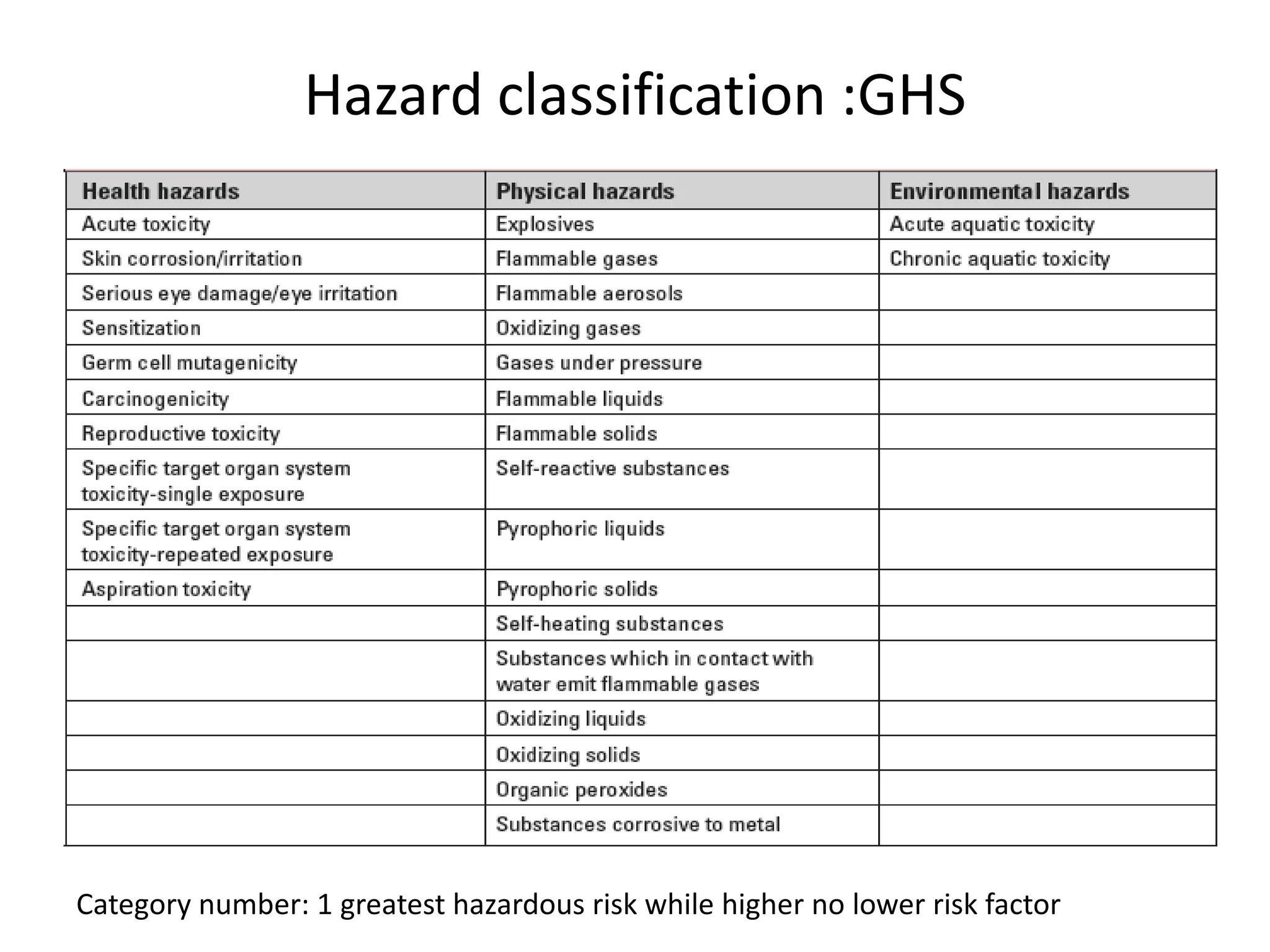
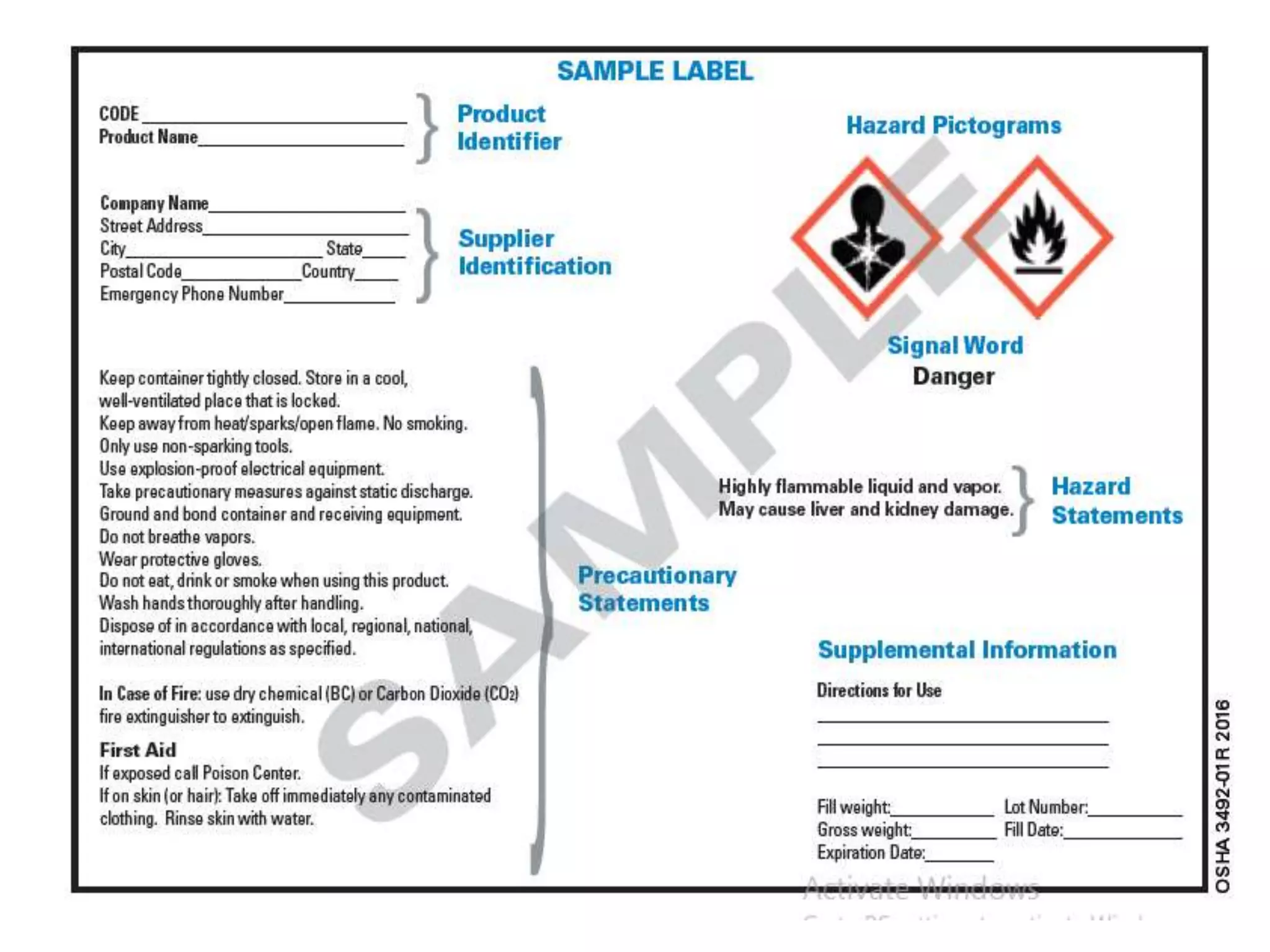
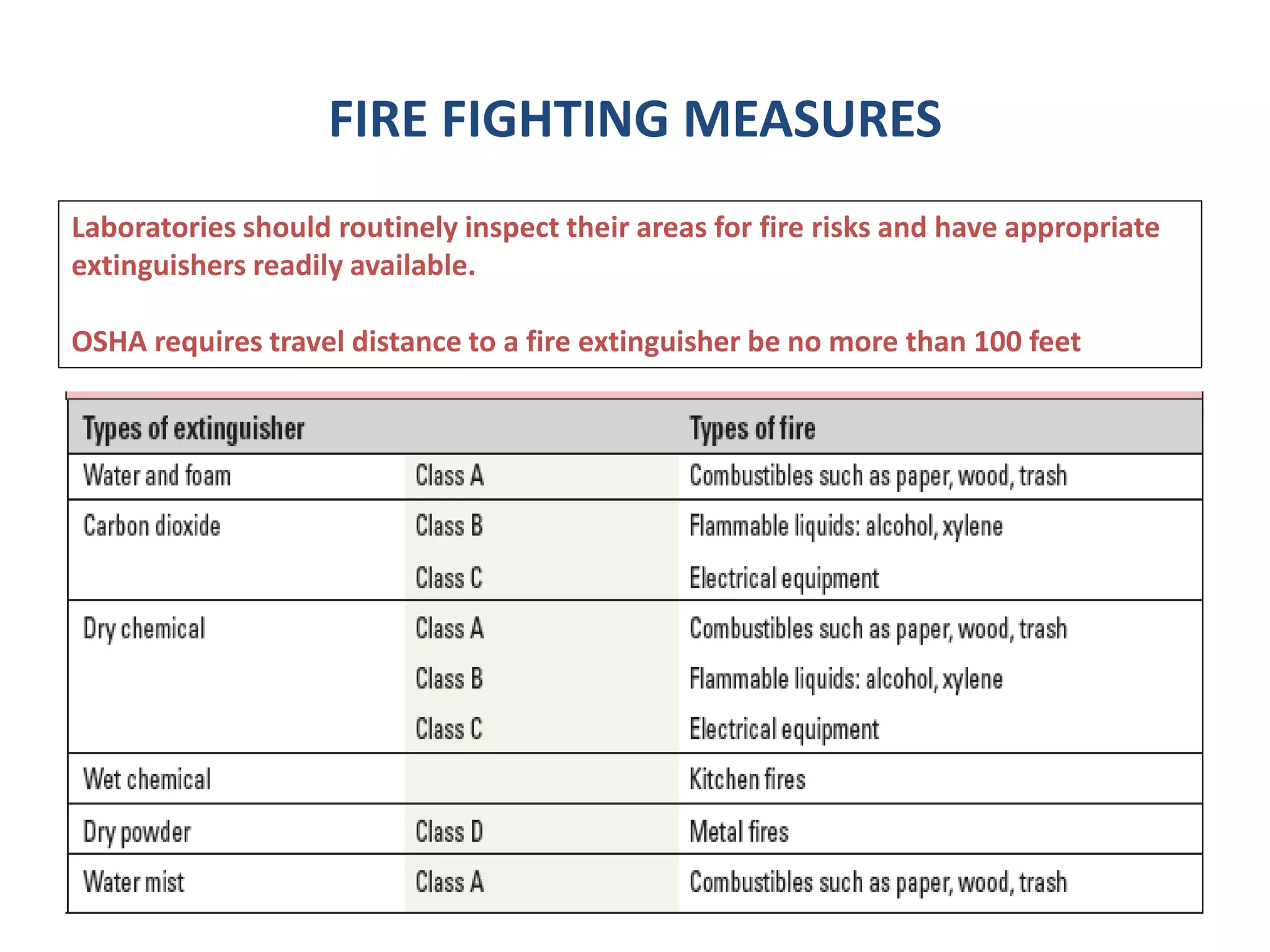
The document outlines the occupational hazards faced by workers in pathology laboratories, emphasizing chemical safety and the importance of adhering to guidelines set by OSHA and GHS. It details various chemicals used in histopathology, their risks, labeling requirements, and proper handling, storage, and disposal procedures to mitigate exposure and ensure safety. Key points include the importance of personal protective equipment (PPE), spill response protocols, and the specific health risks associated with commonly used substances like formaldehyde, isopropanol, and xylene.












![ACCIDENTAL RELEASE MEASURES
• Laboratory must be prepared with all the specific spill cleanup
supplies (personal protective equipment [PPE], absorbents,
neutralizers)
• Size of a spill that will determine the cleanup procedure
• Characteristics of the chemical and ventilation play a major
role
• Spill response procedure must be developed to cover every
chemical used in the laboratory
• The written procedure must document evacuation routes and
alarms in case they are needed.](https://image.slidesharecdn.com/chemicalsafety-191015154600/75/Chemical-safety-in-histopathology-lab-17-2048.jpg)