Embed presentation
Downloaded 17 times



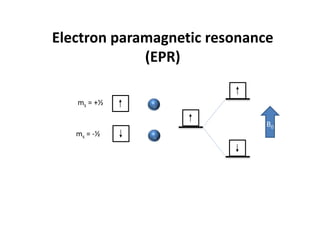

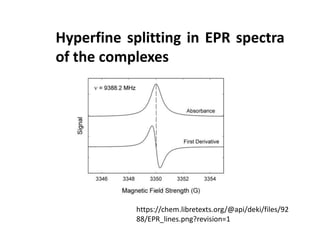
This document discusses evidence for covalent bonding in metal complexes from three perspectives: 1. The nephelauxetic effect shows that electron-electron repulsion is less in complexes than free metal ions due to delocalization of electrons over ligand orbitals. 2. Nephelauxetic parameters (β) quantify this effect, with softer ligands having smaller β values. 3. Electron paramagnetic resonance (EPR) spectroscopy reveals hyperfine splitting in complex spectra, showing interaction between ligand nuclear spins and metal electron spins, further indicating covalent bonding.





