Parts of the periodic table
•Download as DOCX, PDF•
0 likes•340 views
The document is a 15 question quiz about parts of the periodic table. It tests knowledge about where different groups of elements are located, including metals on the left side, transitional metals in the middle, and metalloids between metals and non-metals. It also asks about the properties of metals and the inventor of the periodic table. The questions cover topics like atomic structure, isotopes, ions, and how to interpret the numbers in periodic table cells that indicate protons, neutrons, and atomic mass.
Report
Share
Report
Share
Recommended
Attacking the TEKS: Bonding

Attacking the TEKS: Focus on Bonding presented by Claudia Wallace, ACT2 2010
This session will expose you to the new TEKS and College Readiness Standards. Ideas for sequencing and planning the unit will be shared along with tips for appropriate demos, labs, and assessments. The intended audience is for teachers with 3 or less years of experience or anyone who wants to delve deeper into the new standards.
Recommended
Attacking the TEKS: Bonding

Attacking the TEKS: Focus on Bonding presented by Claudia Wallace, ACT2 2010
This session will expose you to the new TEKS and College Readiness Standards. Ideas for sequencing and planning the unit will be shared along with tips for appropriate demos, labs, and assessments. The intended audience is for teachers with 3 or less years of experience or anyone who wants to delve deeper into the new standards.
Unit checks chemistry1 questions

These questions accompany the ppt. presentation entitled
Chemistry 1: Introduction to Atoms and Periodic Table by Robin D. Seamon
Class 10. Chapter 5. Periodic Classification of Elements

A high quality content of science.
Class X Science
More Related Content
What's hot
Unit checks chemistry1 questions

These questions accompany the ppt. presentation entitled
Chemistry 1: Introduction to Atoms and Periodic Table by Robin D. Seamon
Class 10. Chapter 5. Periodic Classification of Elements

A high quality content of science.
Class X Science
What's hot (20)
Science presentation on periodic classification of elements

Science presentation on periodic classification of elements
Class 10. Chapter 5. Periodic Classification of Elements

Class 10. Chapter 5. Periodic Classification of Elements
Similar to Parts of the periodic table
Test bank for chemistry atoms first 2nd edition by burdge

Test Bank for Chemistry Atoms First 2nd Edition by Burdge
Full download: https://goo.gl/Z56fCq
People also search:
chemistry atoms first 3rd edition pdf
general chemistry atoms first pdf free download
general chemistry atoms first 2nd edition pdf
chemistry atoms first book
chemistry atoms first 2nd edition year
general chemistry atoms first 2nd edition solutions pdf
introductory chemistry an atoms first approach pdf
NCERT solutions for class 10 science chapter 5 (Periodic Classification of El...

Answer: The first ten elements in modern periodic table are hydrogen, helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine and ...for more reading click here,https://bidyashramodia.blogspot.com/2022/06/ncert-solutions-for-class-10-science_24.html
Similar to Parts of the periodic table (20)
Test bank for chemistry atoms first 2nd edition by burdge

Test bank for chemistry atoms first 2nd edition by burdge
NCERT solutions for class 10 science chapter 5 (Periodic Classification of El...

NCERT solutions for class 10 science chapter 5 (Periodic Classification of El...
Recently uploaded
The Roman Empire A Historical Colossus.pdf

The Roman Empire, a vast and enduring power, stands as one of history's most remarkable civilizations, leaving an indelible imprint on the world. It emerged from the Roman Republic, transitioning into an imperial powerhouse under the leadership of Augustus Caesar in 27 BCE. This transformation marked the beginning of an era defined by unprecedented territorial expansion, architectural marvels, and profound cultural influence.
The empire's roots lie in the city of Rome, founded, according to legend, by Romulus in 753 BCE. Over centuries, Rome evolved from a small settlement to a formidable republic, characterized by a complex political system with elected officials and checks on power. However, internal strife, class conflicts, and military ambitions paved the way for the end of the Republic. Julius Caesar’s dictatorship and subsequent assassination in 44 BCE created a power vacuum, leading to a civil war. Octavian, later Augustus, emerged victorious, heralding the Roman Empire’s birth.
Under Augustus, the empire experienced the Pax Romana, a 200-year period of relative peace and stability. Augustus reformed the military, established efficient administrative systems, and initiated grand construction projects. The empire's borders expanded, encompassing territories from Britain to Egypt and from Spain to the Euphrates. Roman legions, renowned for their discipline and engineering prowess, secured and maintained these vast territories, building roads, fortifications, and cities that facilitated control and integration.
The Roman Empire’s society was hierarchical, with a rigid class system. At the top were the patricians, wealthy elites who held significant political power. Below them were the plebeians, free citizens with limited political influence, and the vast numbers of slaves who formed the backbone of the economy. The family unit was central, governed by the paterfamilias, the male head who held absolute authority.
Culturally, the Romans were eclectic, absorbing and adapting elements from the civilizations they encountered, particularly the Greeks. Roman art, literature, and philosophy reflected this synthesis, creating a rich cultural tapestry. Latin, the Roman language, became the lingua franca of the Western world, influencing numerous modern languages.
Roman architecture and engineering achievements were monumental. They perfected the arch, vault, and dome, constructing enduring structures like the Colosseum, Pantheon, and aqueducts. These engineering marvels not only showcased Roman ingenuity but also served practical purposes, from public entertainment to water supply.
How libraries can support authors with open access requirements for UKRI fund...

How libraries can support authors with open access requirements for UKRI funded books
Wednesday 22 May 2024, 14:00-15:00.
MARUTI SUZUKI- A Successful Joint Venture in India.pptx

Let us know about Maruti Suzuki, a successful Joint venture in India.
Operation Blue Star - Saka Neela Tara

Operation “Blue Star” is the only event in the history of Independent India where the state went into war with its own people. Even after about 40 years it is not clear if it was culmination of states anger over people of the region, a political game of power or start of dictatorial chapter in the democratic setup.
The people of Punjab felt alienated from main stream due to denial of their just demands during a long democratic struggle since independence. As it happen all over the word, it led to militant struggle with great loss of lives of military, police and civilian personnel. Killing of Indira Gandhi and massacre of innocent Sikhs in Delhi and other India cities was also associated with this movement.
How to Break the cycle of negative Thoughts

We all have good and bad thoughts from time to time and situation to situation. We are bombarded daily with spiraling thoughts(both negative and positive) creating all-consuming feel , making us difficult to manage with associated suffering. Good thoughts are like our Mob Signal (Positive thought) amidst noise(negative thought) in the atmosphere. Negative thoughts like noise outweigh positive thoughts. These thoughts often create unwanted confusion, trouble, stress and frustration in our mind as well as chaos in our physical world. Negative thoughts are also known as “distorted thinking”.
Introduction to Quality Improvement Essentials

This is a presentation by Dada Robert in a Your Skill Boost masterclass organised by the Excellence Foundation for South Sudan (EFSS) on Saturday, the 25th and Sunday, the 26th of May 2024.
He discussed the concept of quality improvement, emphasizing its applicability to various aspects of life, including personal, project, and program improvements. He defined quality as doing the right thing at the right time in the right way to achieve the best possible results and discussed the concept of the "gap" between what we know and what we do, and how this gap represents the areas we need to improve. He explained the scientific approach to quality improvement, which involves systematic performance analysis, testing and learning, and implementing change ideas. He also highlighted the importance of client focus and a team approach to quality improvement.
TESDA TM1 REVIEWER FOR NATIONAL ASSESSMENT WRITTEN AND ORAL QUESTIONS WITH A...

TESDA TM1 REVIEWER FOR NATIONAL ASSESSMENT WRITTEN AND ORAL QUESTIONS WITH ANSWERS.
1.4 modern child centered education - mahatma gandhi-2.pptx

Child centred education is an educational approach that priorities the interest, needs and abilities of the child in the learning process.
Welcome to TechSoup New Member Orientation and Q&A (May 2024).pdf

In this webinar you will learn how your organization can access TechSoup's wide variety of product discount and donation programs. From hardware to software, we'll give you a tour of the tools available to help your nonprofit with productivity, collaboration, financial management, donor tracking, security, and more.
Overview on Edible Vaccine: Pros & Cons with Mechanism

This ppt include the description of the edible vaccine i.e. a new concept over the traditional vaccine administered by injection.
2024.06.01 Introducing a competency framework for languag learning materials ...

http://sandymillin.wordpress.com/iateflwebinar2024
Published classroom materials form the basis of syllabuses, drive teacher professional development, and have a potentially huge influence on learners, teachers and education systems. All teachers also create their own materials, whether a few sentences on a blackboard, a highly-structured fully-realised online course, or anything in between. Despite this, the knowledge and skills needed to create effective language learning materials are rarely part of teacher training, and are mostly learnt by trial and error.
Knowledge and skills frameworks, generally called competency frameworks, for ELT teachers, trainers and managers have existed for a few years now. However, until I created one for my MA dissertation, there wasn’t one drawing together what we need to know and do to be able to effectively produce language learning materials.
This webinar will introduce you to my framework, highlighting the key competencies I identified from my research. It will also show how anybody involved in language teaching (any language, not just English!), teacher training, managing schools or developing language learning materials can benefit from using the framework.
The geography of Taylor Swift - some ideas

Geographical themes connected with Taylor Swift's ERAS tour - coming to the UK in June 2024
How to Split Bills in the Odoo 17 POS Module

Bills have a main role in point of sale procedure. It will help to track sales, handling payments and giving receipts to customers. Bill splitting also has an important role in POS. For example, If some friends come together for dinner and if they want to divide the bill then it is possible by POS bill splitting. This slide will show how to split bills in odoo 17 POS.
Sectors of the Indian Economy - Class 10 Study Notes pdf

The Indian economy is classified into different sectors to simplify the analysis and understanding of economic activities. For Class 10, it's essential to grasp the sectors of the Indian economy, understand their characteristics, and recognize their importance. This guide will provide detailed notes on the Sectors of the Indian Economy Class 10, using specific long-tail keywords to enhance comprehension.
For more information, visit-www.vavaclasses.com
How to Create Map Views in the Odoo 17 ERP

The map views are useful for providing a geographical representation of data. They allow users to visualize and analyze the data in a more intuitive manner.
Instructions for Submissions thorugh G- Classroom.pptx

This presentation provides a briefing on how to upload submissions and documents in Google Classroom. It was prepared as part of an orientation for new Sainik School in-service teacher trainees. As a training officer, my goal is to ensure that you are comfortable and proficient with this essential tool for managing assignments and fostering student engagement.
CLASS 11 CBSE B.St Project AIDS TO TRADE - INSURANCE

Class 11 CBSE Business Studies Project ( AIDS TO TRADE - INSURANCE)
Recently uploaded (20)
How libraries can support authors with open access requirements for UKRI fund...

How libraries can support authors with open access requirements for UKRI fund...
MARUTI SUZUKI- A Successful Joint Venture in India.pptx

MARUTI SUZUKI- A Successful Joint Venture in India.pptx
TESDA TM1 REVIEWER FOR NATIONAL ASSESSMENT WRITTEN AND ORAL QUESTIONS WITH A...

TESDA TM1 REVIEWER FOR NATIONAL ASSESSMENT WRITTEN AND ORAL QUESTIONS WITH A...
1.4 modern child centered education - mahatma gandhi-2.pptx

1.4 modern child centered education - mahatma gandhi-2.pptx
Welcome to TechSoup New Member Orientation and Q&A (May 2024).pdf

Welcome to TechSoup New Member Orientation and Q&A (May 2024).pdf
Overview on Edible Vaccine: Pros & Cons with Mechanism

Overview on Edible Vaccine: Pros & Cons with Mechanism
2024.06.01 Introducing a competency framework for languag learning materials ...

2024.06.01 Introducing a competency framework for languag learning materials ...
Sectors of the Indian Economy - Class 10 Study Notes pdf

Sectors of the Indian Economy - Class 10 Study Notes pdf
Instructions for Submissions thorugh G- Classroom.pptx

Instructions for Submissions thorugh G- Classroom.pptx
Basic phrases for greeting and assisting costumers

Basic phrases for greeting and assisting costumers
CLASS 11 CBSE B.St Project AIDS TO TRADE - INSURANCE

CLASS 11 CBSE B.St Project AIDS TO TRADE - INSURANCE
Parts of the periodic table
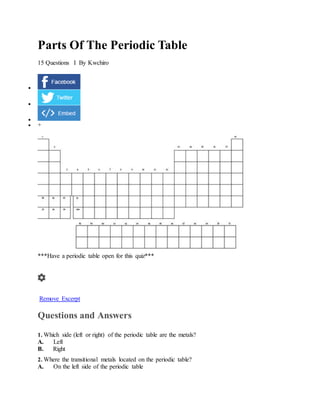
- 1. Parts Of The Periodic Table 15 Questions I By Kwchiro + ***Have a periodic table open for this quiz*** Remove Excerpt Questions and Answers 1. Which side (left or right) of the periodic table are the metals? A. Left B. Right 2. Where the transitional metals located on the periodic table? A. On the left side of the periodic table
- 2. B. In the middle of the peridic table C. On the right side of the periodic table. 3. Who invented the periodic table? A. Ernst Rutherford B. Henry Mosely C. Dimitri Mendeleev 4. Which direction (up/down or left/right) are the families? A. Left/right B. Up/down 5. Which element is located in group 2, period 3? A. Be B. Mg C. B 6. How many electrons can fit in the first energy level? A. 2 B. 8 C. 18 7. What does the number 3 indicate in this cell from the periodic table? A. Number of neutrons in a lithium atom B. The atomic mass in the lithium atom C. The number of protons in the lithium atom 8. What does the number 3.91 indicate in this cell form the periodic table? A. 3.91 indicates the mass of 1 atom of lithium B. Indicates the average atomic mass of lithium - taking into consideration the naturally occuring isotopes C. It is the average number of protons in lithium metals 9. Which are characteristics of metals? A. Conduct electricity and heat. B. Non-shiny and generally liquid at room temperature. C. Shiny and generally gas at room temperature. 10. Where are the metalloids? A. They are the bottom two periods on the periodic table. B. They are the first family of metals C. They are the elements that straddle the step-like like between the metals and non-metals. They share characteristics of both groups. 11. Which group of elements are called the Alkaline metals? A. Group 1 (IA) B. Group 2 (IIA) C. Group 3 (IIIA) D. Group7 (VIIA or 17) E. Group 8 (VIIIA or 18) 12. Which element from the periodic table would this correspond with? (Assuming the same
- 3. number of protons as electrons). A. 6 electrons and 6 protons means carbon B. 4 valence electrons indicates it is beryllium C. None of the above 13. Which element would have 20 protons and 19 neutrons? A. Potassium B. Calcium C. Sodium 14. Which of the following indicates an ion? A. An atom with 6 protons, 6 electrons, but only 5 neutrons B. An atom with 6 protons, 6 electrons and 6 neutrons C. An elements with 6 protons, 5 electrons and 5 neutrons 15. Which of the following are isotopes? A. Two carbon atoms, one with 6 electrons, one with only 5 electrons. B. Two oxygen atoms, one with 6 neutrons and 1 with 5 neutrons C. An oxygen atom with 6 neutrons and a nitrogen atom with 6 neutrons
