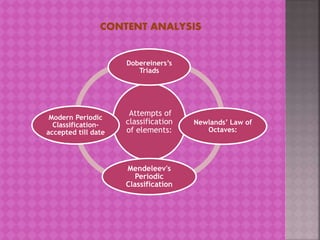
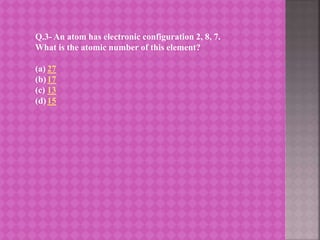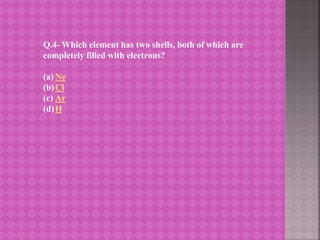The document provides learning material on the periodic classification of elements. It discusses early attempts at classifying elements, including Dobereiner's triads, Newlands' law of octaves, and Mendeleev's periodic table. It outlines the objectives of understanding periodic classification, analyzing early classification attempts, and being able to design the modern periodic table based on element properties. It presents multiple choice questions to test understanding of trends in the periodic table, properties predicted by group membership, and electronic configurations of elements.