The Periodic Table Presentation 1
•Download as PPT, PDF•
21 likes•8,553 views
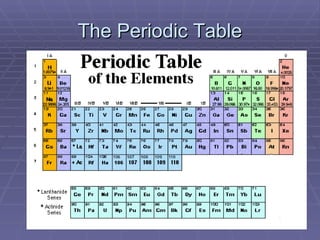
The document provides an overview of the development of the periodic table. It discusses early chemists like Lavoisier who compiled lists of known elements. In the 1860s, Newlands and Meyer began to notice patterns in properties of elements when arranged by atomic mass. Mendeleev later created the first periodic table by arranging elements in order of atomic mass with similar properties grouped together. This table had some issues resolved by Moseley in 1913, who arranged elements by atomic number, establishing the modern periodic table and periodic law.
Report
Share
Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Viewers also liked
Viewers also liked (20)
The periodic table presentation for 4050 [autosaved]![The periodic table presentation for 4050 [autosaved]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![The periodic table presentation for 4050 [autosaved]](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
The periodic table presentation for 4050 [autosaved]
Moseley - His contribution To Physics and Chemistry

Moseley - His contribution To Physics and Chemistry
Similar to The Periodic Table Presentation 1
Similar to The Periodic Table Presentation 1 (20)
Periodic table of chemical elements impact on human welfare

Periodic table of chemical elements impact on human welfare
Chemistry Unit 2 Part 4 - Development and Organization of the Periodic Table

Chemistry Unit 2 Part 4 - Development and Organization of the Periodic Table
More from itamarita1984
More from itamarita1984 (20)
Recently uploaded
Recently uploaded (20)
2024: Domino Containers - The Next Step. News from the Domino Container commu...

2024: Domino Containers - The Next Step. News from the Domino Container commu...
08448380779 Call Girls In Civil Lines Women Seeking Men

08448380779 Call Girls In Civil Lines Women Seeking Men
Boost PC performance: How more available memory can improve productivity

Boost PC performance: How more available memory can improve productivity
Tata AIG General Insurance Company - Insurer Innovation Award 2024

Tata AIG General Insurance Company - Insurer Innovation Award 2024
TrustArc Webinar - Stay Ahead of US State Data Privacy Law Developments

TrustArc Webinar - Stay Ahead of US State Data Privacy Law Developments
Exploring the Future Potential of AI-Enabled Smartphone Processors

Exploring the Future Potential of AI-Enabled Smartphone Processors
IAC 2024 - IA Fast Track to Search Focused AI Solutions

IAC 2024 - IA Fast Track to Search Focused AI Solutions
Handwritten Text Recognition for manuscripts and early printed texts

Handwritten Text Recognition for manuscripts and early printed texts
Understanding Discord NSFW Servers A Guide for Responsible Users.pdf

Understanding Discord NSFW Servers A Guide for Responsible Users.pdf
Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024

Bajaj Allianz Life Insurance Company - Insurer Innovation Award 2024
Powerful Google developer tools for immediate impact! (2023-24 C)

Powerful Google developer tools for immediate impact! (2023-24 C)
The Periodic Table Presentation 1
- 1. The Periodic Table Part One
- 17. Problem-solving lab p 155