Embed presentation
Download to read offline


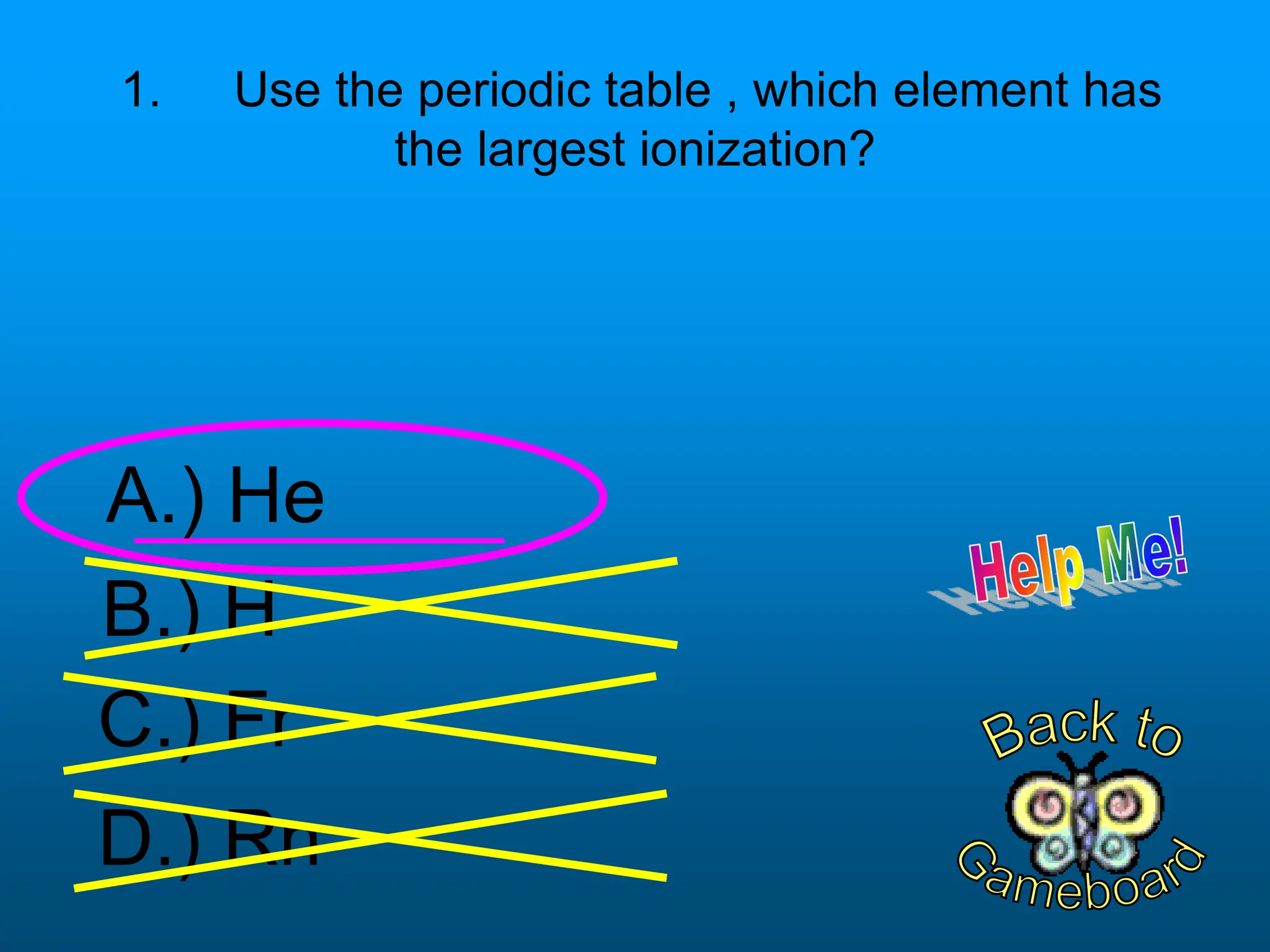
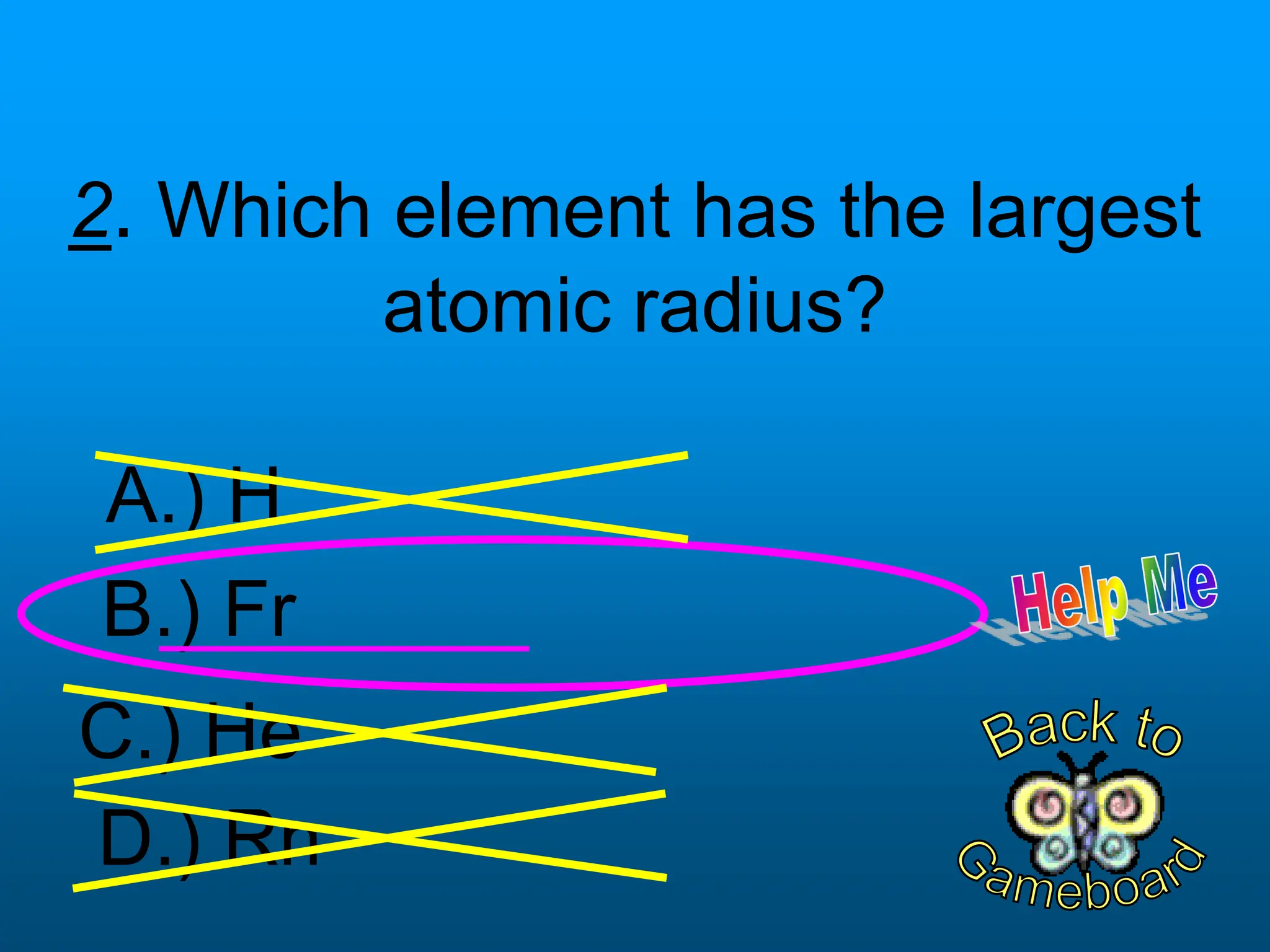
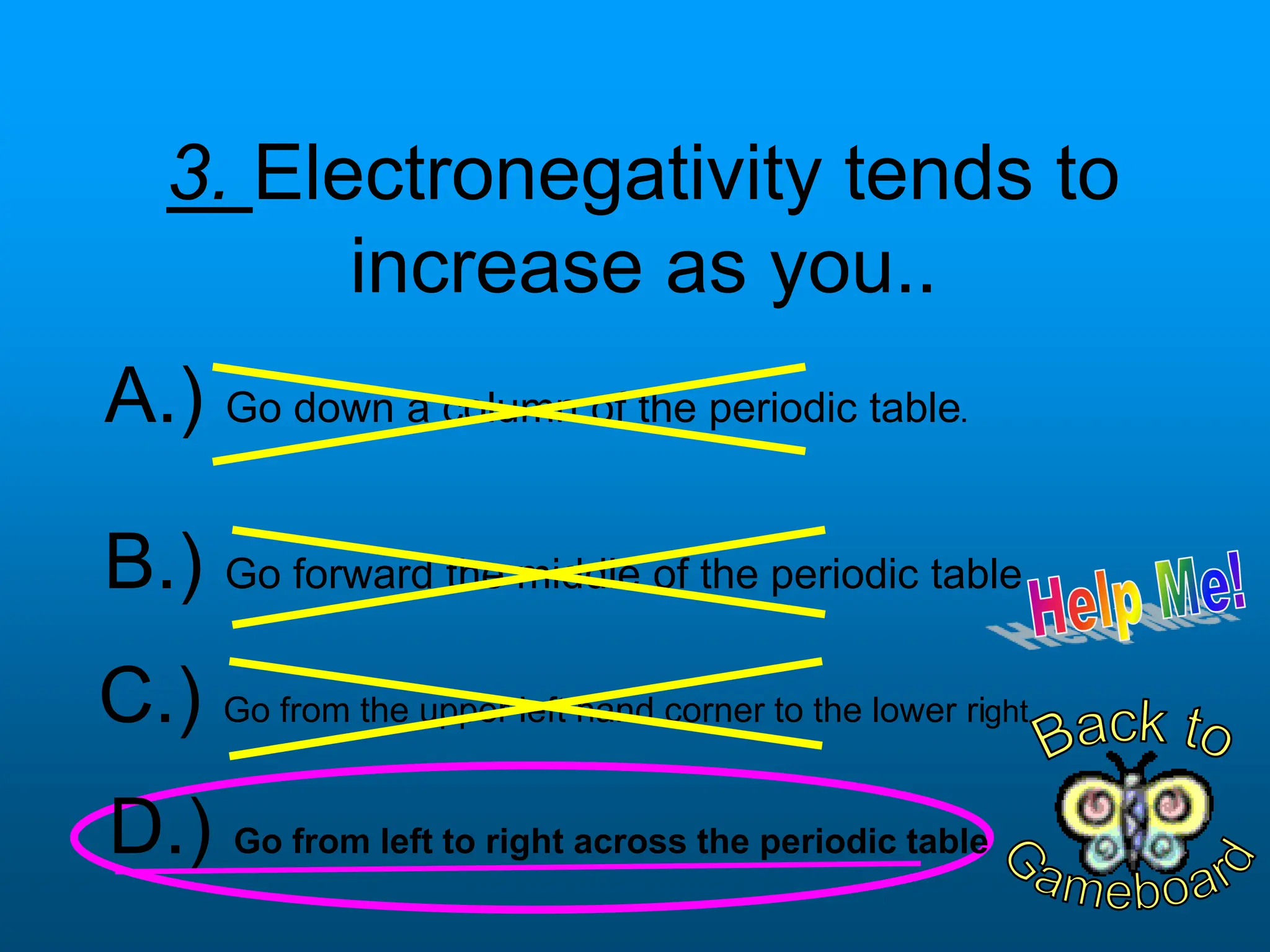
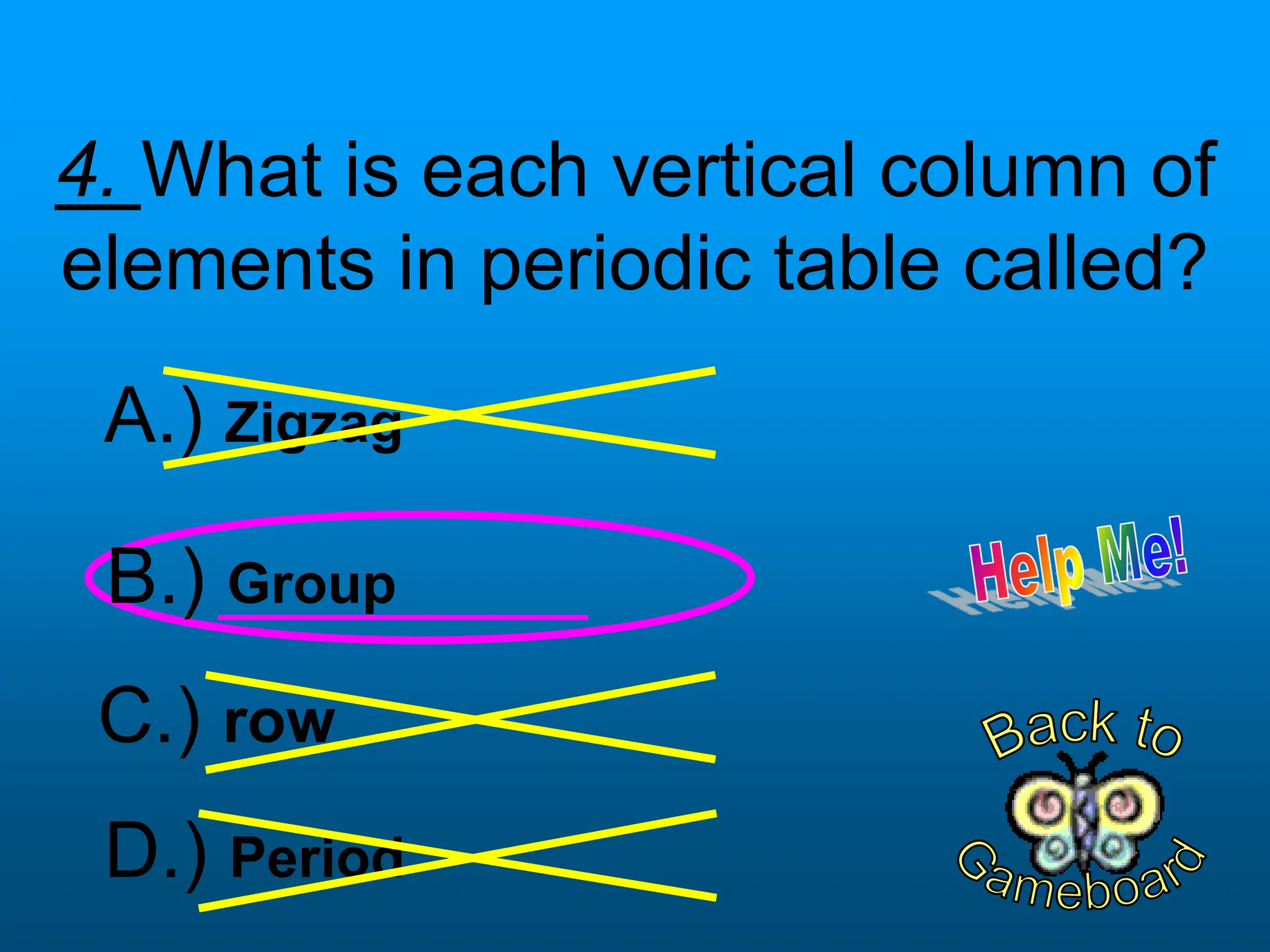
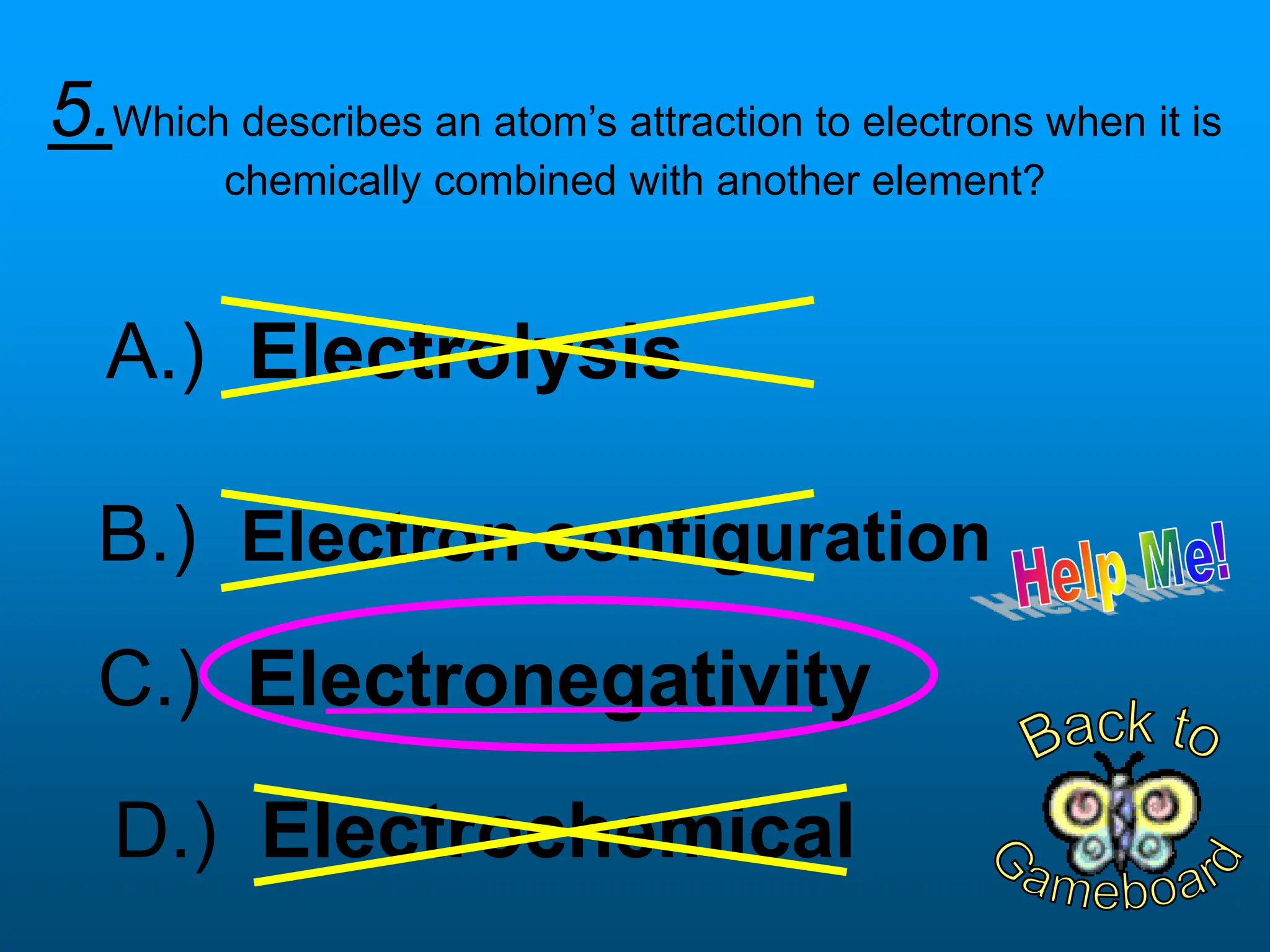

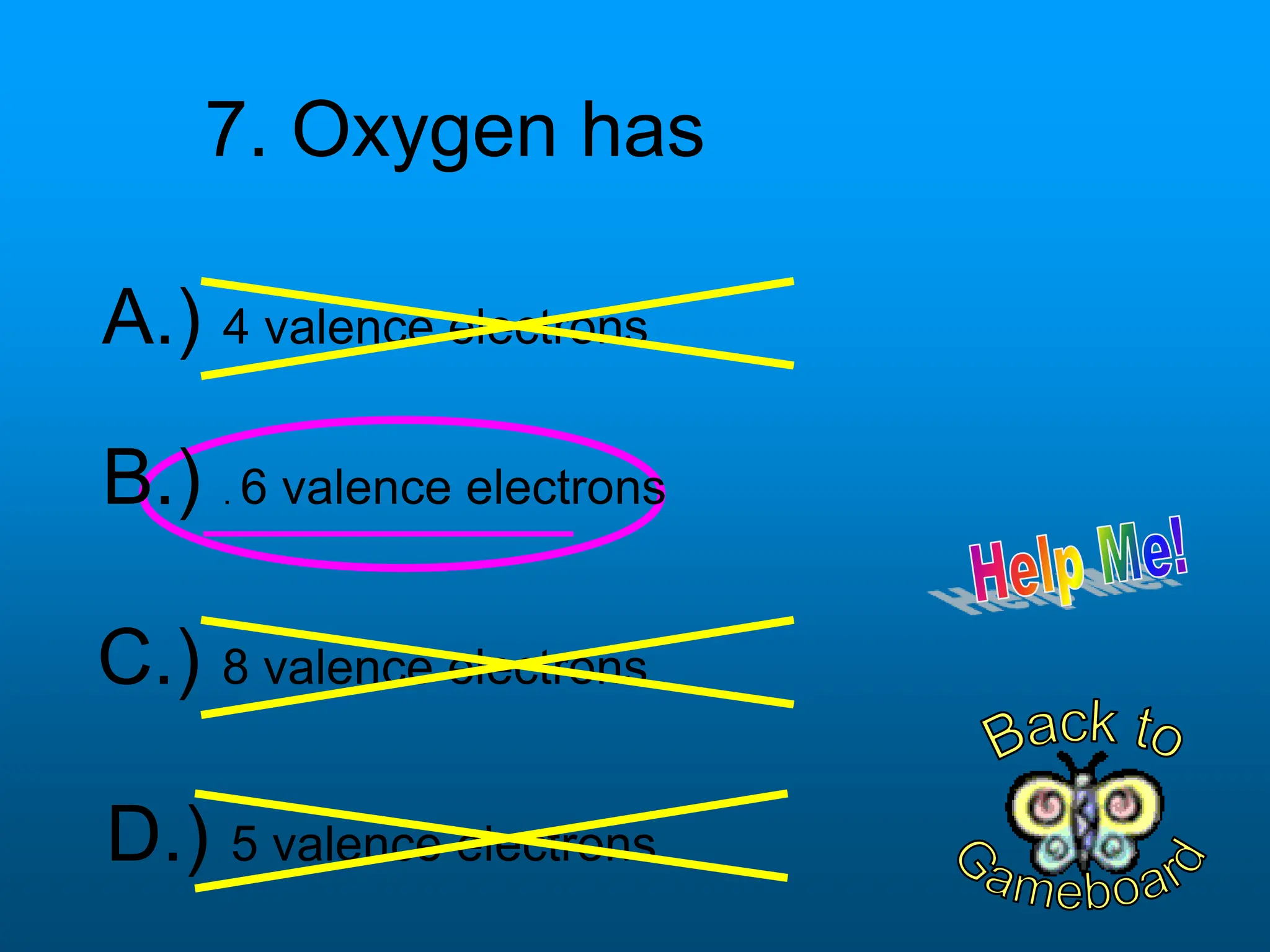
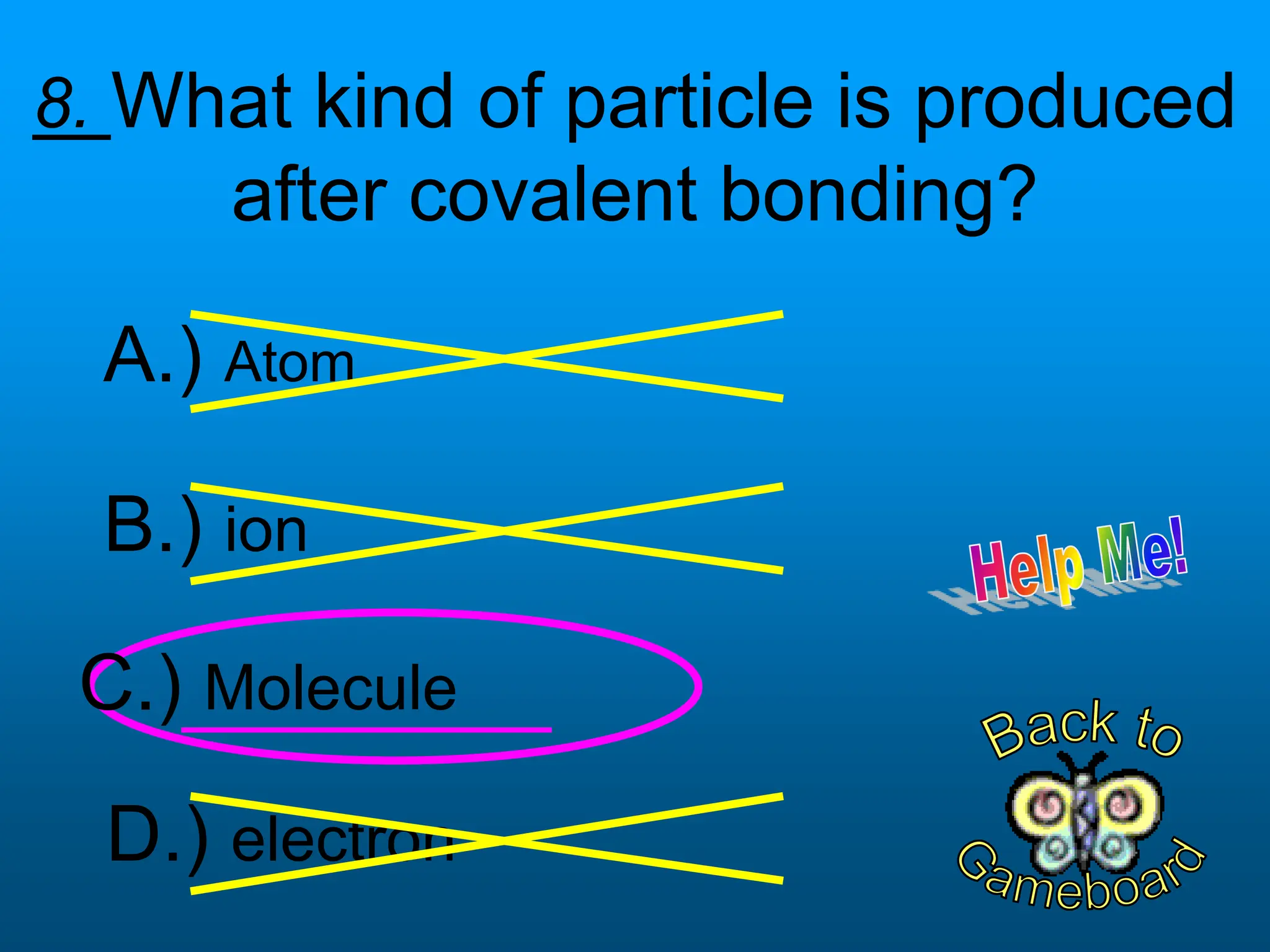
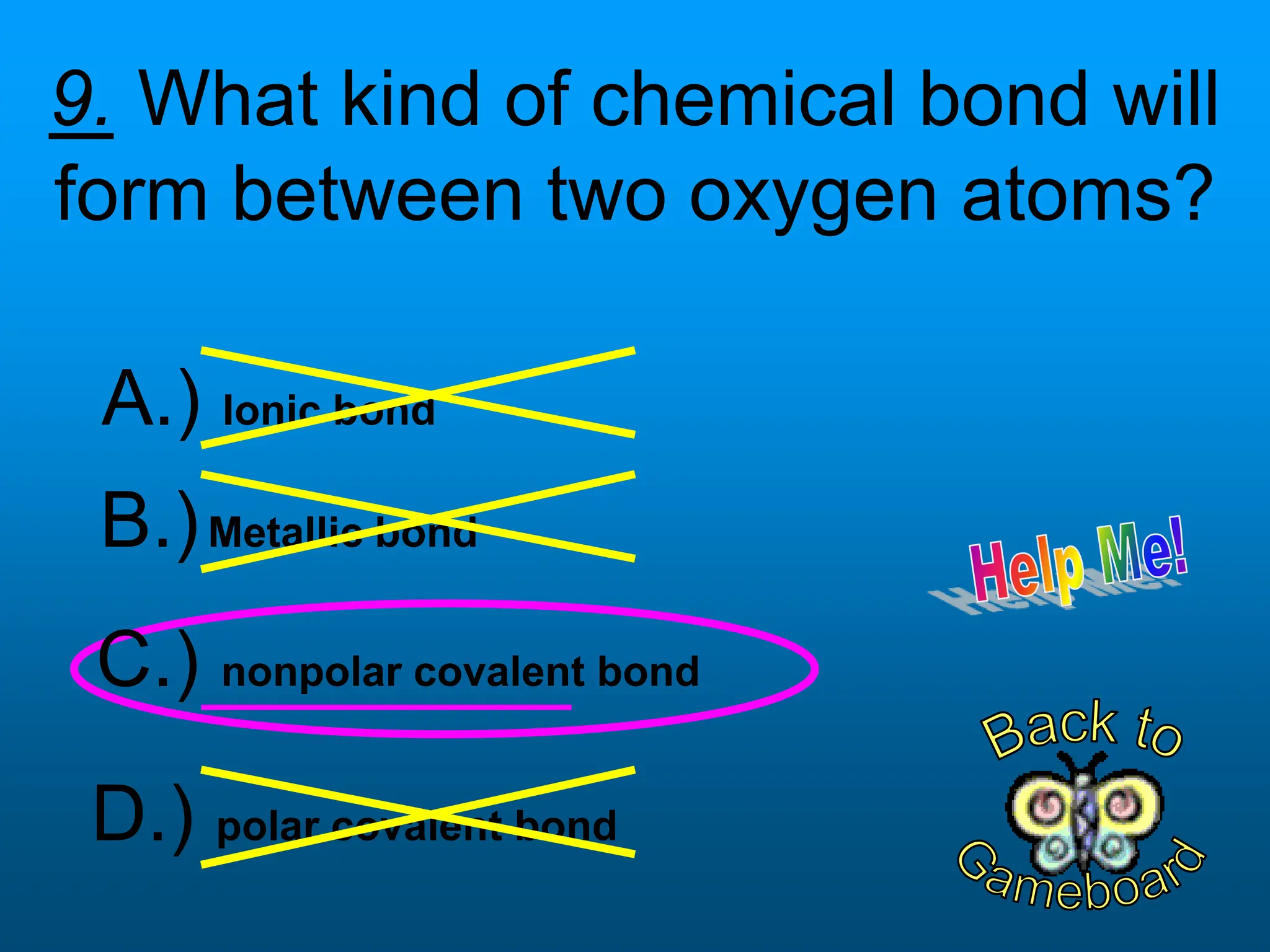

The document contains a short quiz about basic chemistry concepts related to the periodic table, elements, chemical bonds, and valence electrons. It asks 9 multiple choice questions about ionization energy, atomic radius, trends in electronegativity across the periodic table, the name of columns in the periodic table, the definition of electronegativity, the number of valence electrons for carbon and oxygen, the particle produced after covalent bonding, and the type of bond that forms between two oxygen atoms.











