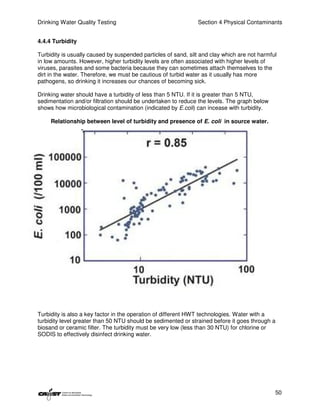
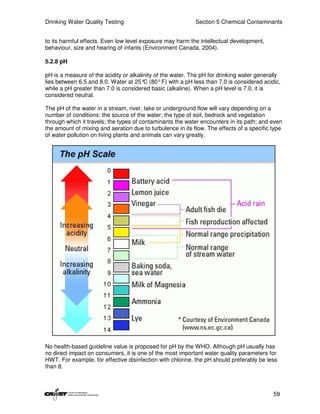
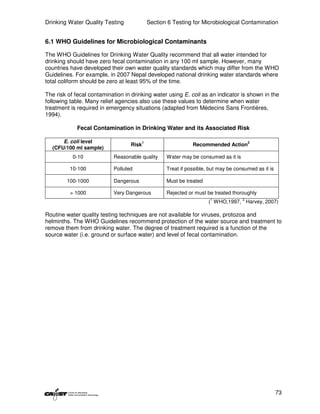
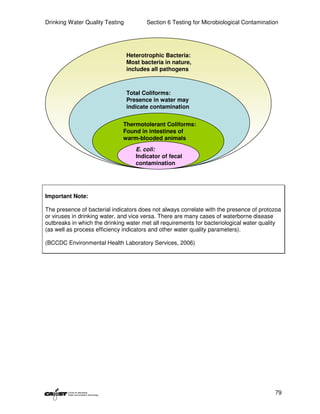
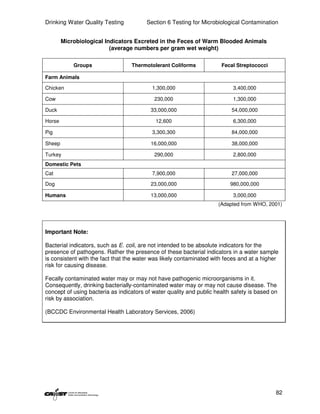
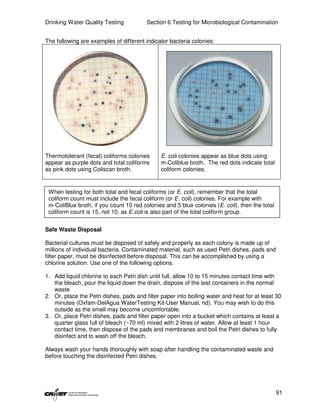
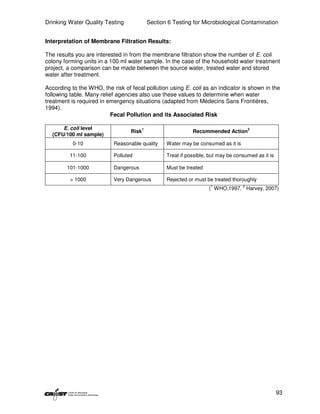
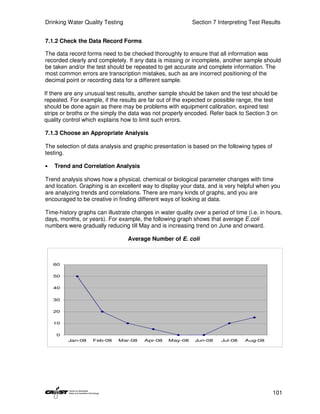
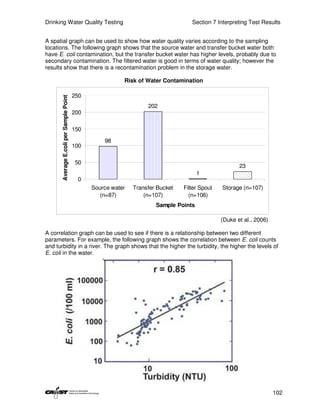
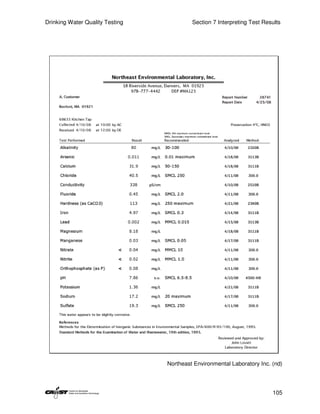
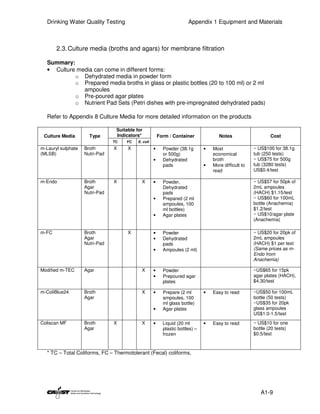
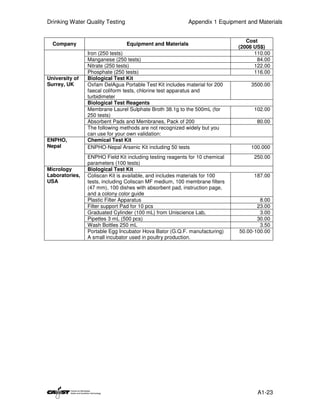
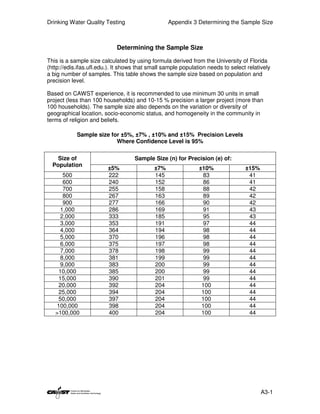
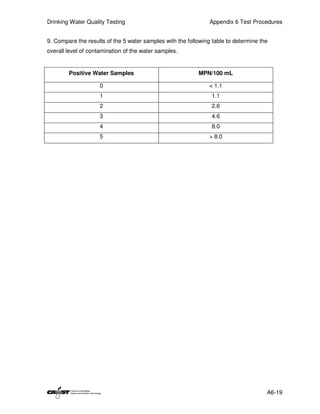
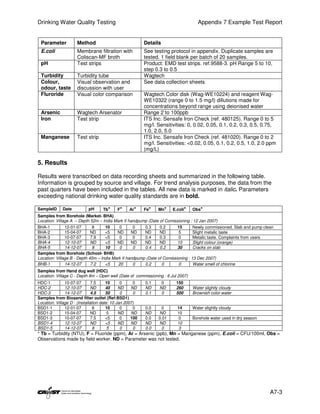
This document provides an introduction to drinking water quality testing. It discusses the global issues of lack of access to safe drinking water and sanitation. Nearly 900 million people lack access to improved drinking water and over 2.5 billion lack access to improved sanitation. Testing drinking water quality is important to ensure water is safe for drinking and meets World Health Organization guidelines. The document outlines the United Nations Millennium Development Goals related to increasing access to safe drinking water and sanitation.