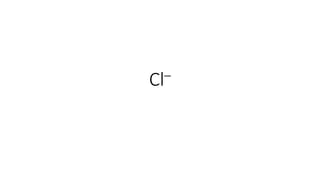
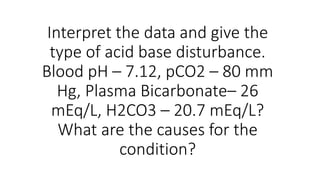
The document discusses various case studies related to acid-base disorders, identifying conditions such as metabolic acidosis and respiratory alkalosis based on arterial blood gas values. It also includes questions on physiological buffering systems, the anion gap, and electrolyte distribution in intracellular versus extracellular fluid. Additionally, it highlights clinical scenarios impacting acid-base status and the underlying causes of specific conditions.