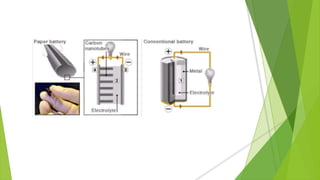
Paper batteries are made largely of cellulose (from paper) and carbon nanotubes. They produce electricity through chemical reactions between a lithium anode, carbon nanotube cathode, and an electrolyte like urine or blood, similar to lithium-ion batteries. Paper batteries are constructed by coating a paper substrate with an ionic solution, spreading a carbon nanotube ink over it, and laminating a thin lithium film on the other side. The paper battery is flexible, biodegradable, and has applications in wearable devices, medical sensors, and reducing electricity demand.