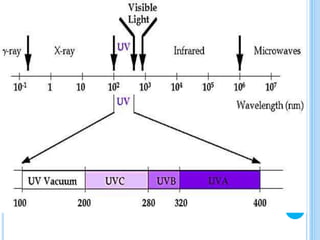
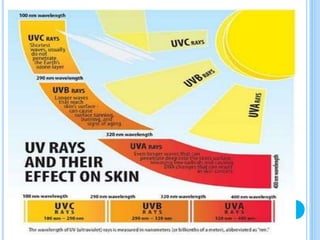
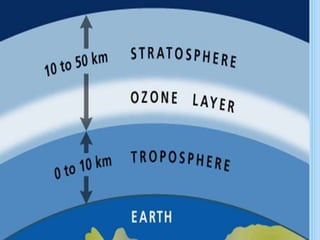
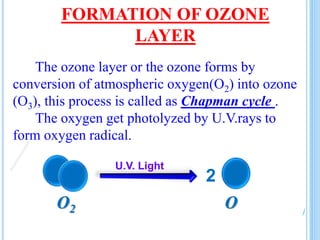
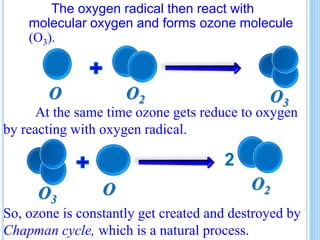
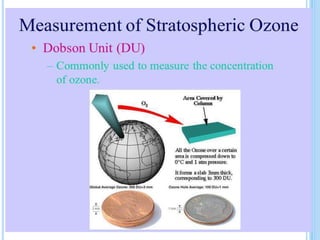
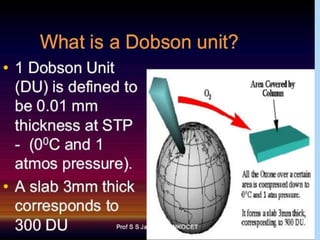
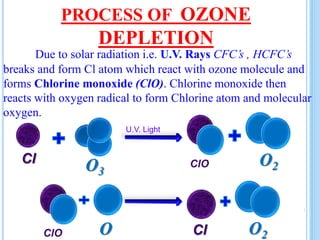
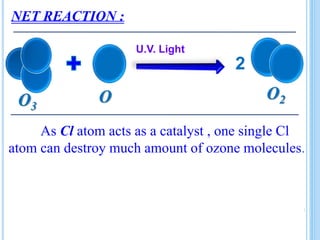
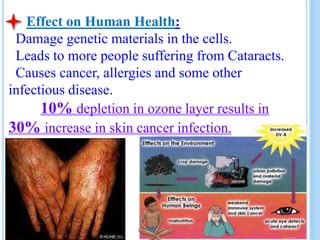
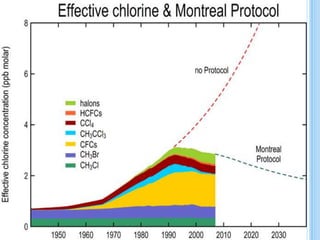
The ozone layer is a layer in the stratosphere between 20-30 km above the Earth's surface that contains 90% of the atmosphere's ozone. Ozone is formed through the Chapman cycle where oxygen is converted to ozone via ultraviolet radiation. Ozone depletion occurs when gases like CFCs and halons react with ozone due to UV radiation, forming chlorine atoms that can destroy 100,000 ozone molecules each. This causes thinning of the ozone layer, known as the ozone hole over Antarctica. Ozone depletion leads to increased UV radiation reaching the Earth, harming humans, animals, plants and contributing to global warming. International agreements like the Montreal Protocol aim to phase out ozone depleting