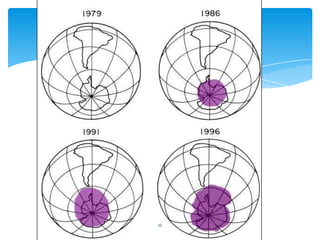
The document summarizes information about the ozone layer and its depletion. It discusses how the ozone layer protects the Earth from ultraviolet radiation from the sun. It then explains how chlorofluorocarbons (CFCs) were depleting the ozone layer when they were released into the atmosphere. CFCs break down ozone molecules in the stratosphere. The document also outlines observations that show the ozone layer depletion peaked around 2010 and is expected to fully recover by 2065 as CFC use has been restricted by the Montreal Protocol.