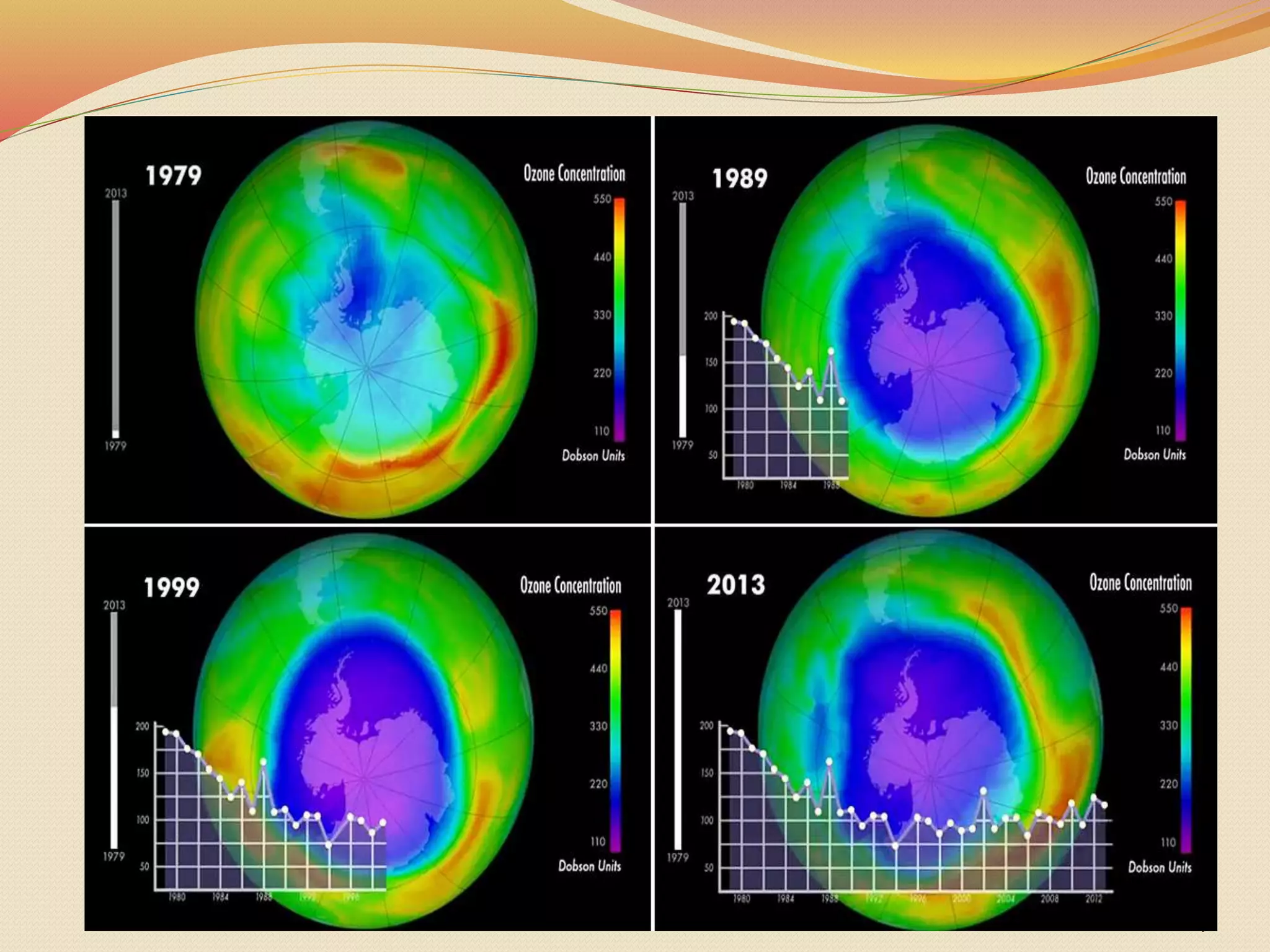
This document discusses the ozone layer and ozone depletion. It begins by introducing the student's name and department. It then provides background on the ozone layer, noting that most ozone is found in the stratosphere between 19-30km, forming the ozone layer.
The document discusses the layers of the atmosphere and the roles of the troposphere, stratosphere, mesosphere, thermosphere, and exosphere. It explains that the ozone layer in the stratosphere absorbs harmful UV rays from the sun. The document also covers causes of ozone depletion like CFCs, effects of ozone depletion like skin cancer, and international agreements to regulate ozone-depleting chemicals like the Montreal