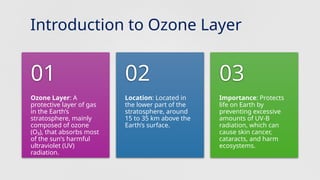
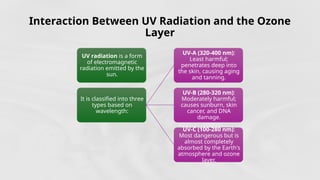
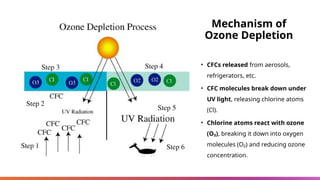
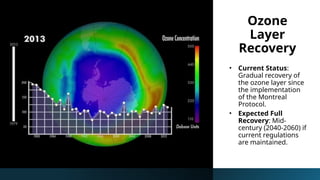
The document discusses the ozone layer, its location, structure, and crucial role in protecting Earth from harmful ultraviolet radiation. It details the causes and effects of ozone depletion due to human-made chemicals, particularly chlorofluorocarbons (CFCs), and highlights the global response through the Montreal Protocol to phase out these substances. The current status indicates a gradual recovery of the ozone layer with expectations for full recovery by the mid-century if regulations are maintained.