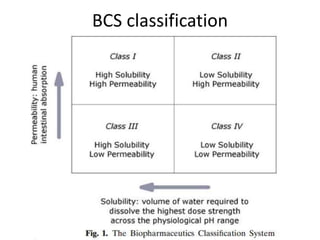
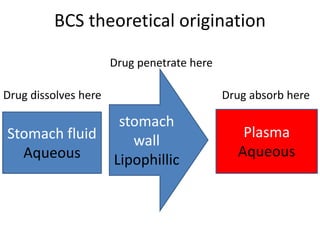
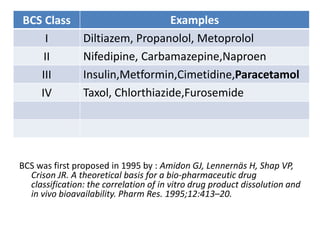
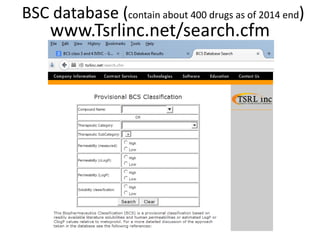
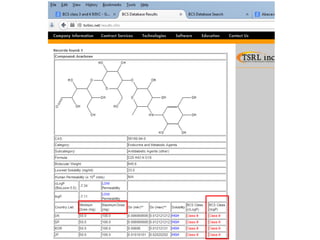
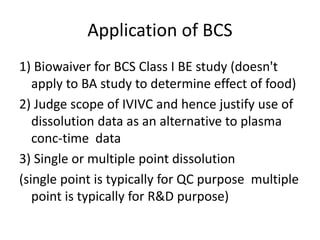
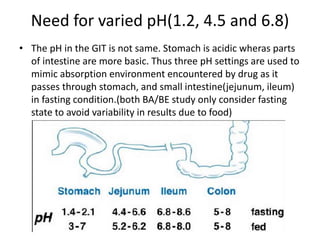
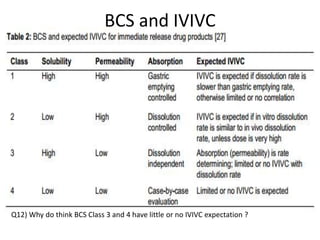
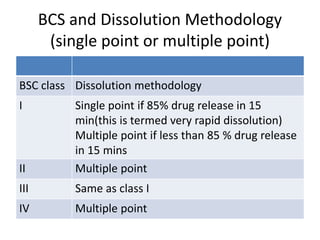
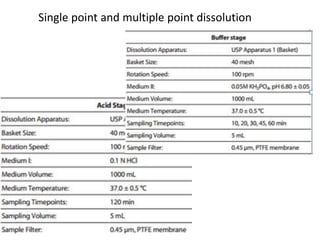
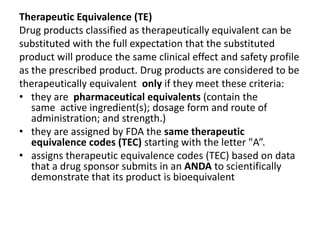
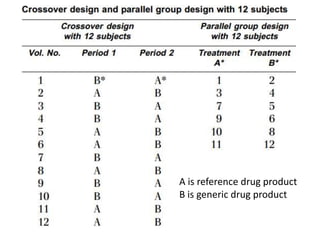
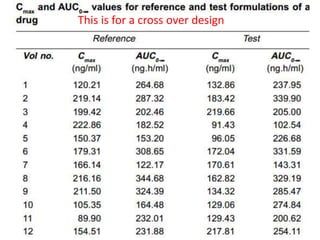
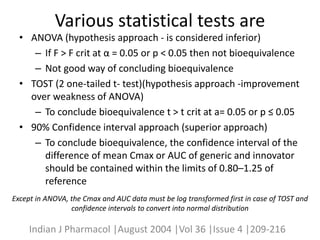
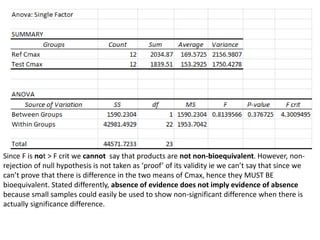
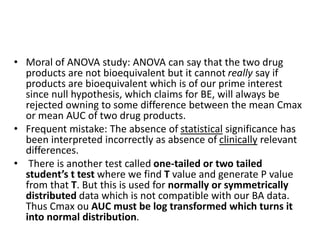
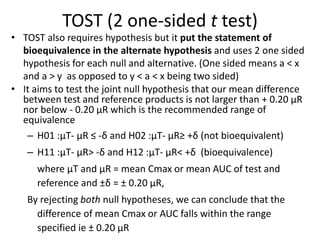
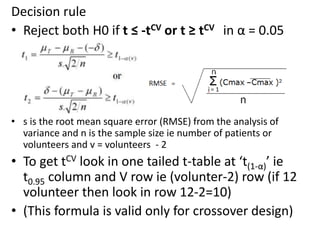
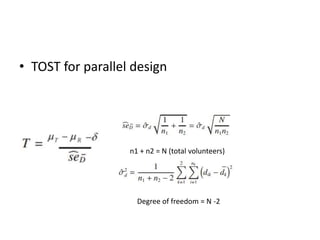
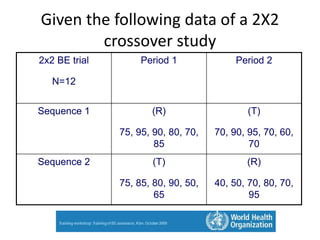
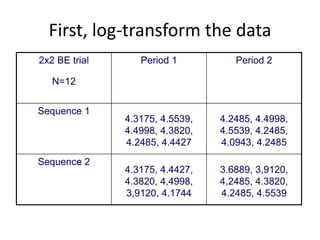
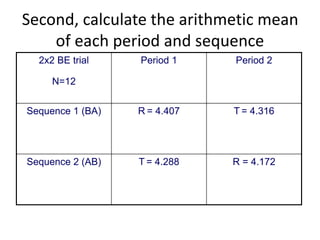
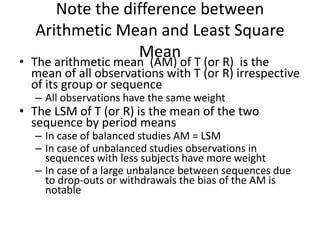
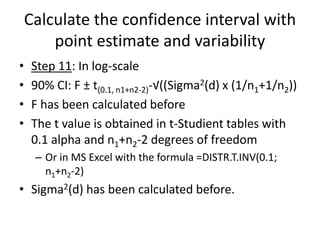
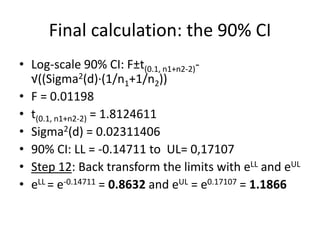
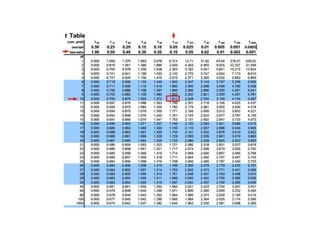
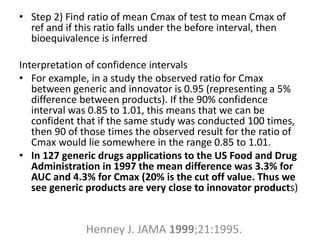
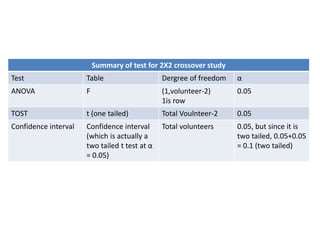
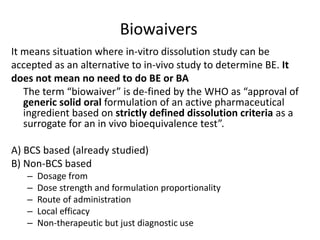
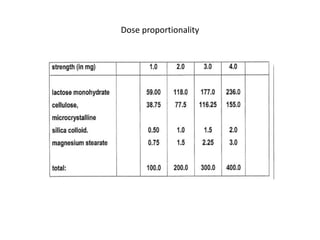
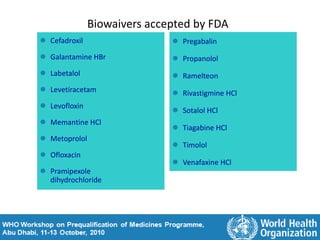
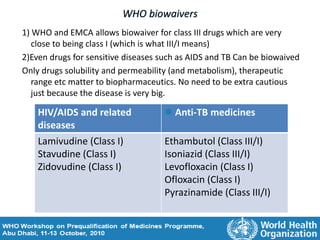
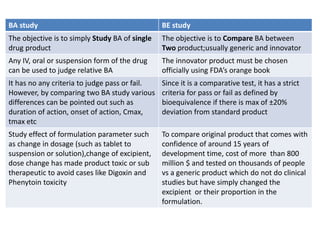
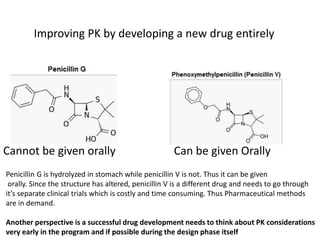
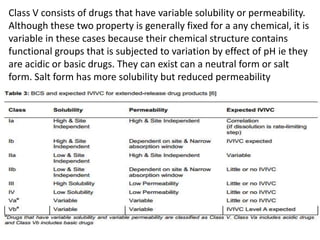
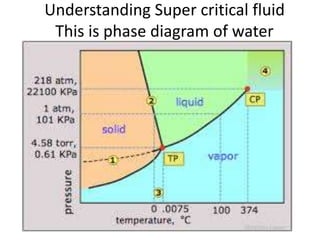
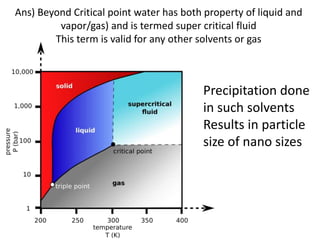
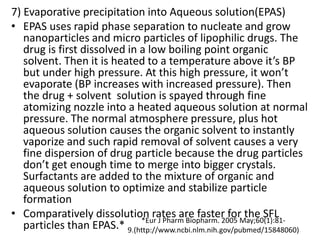
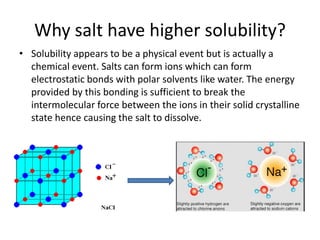
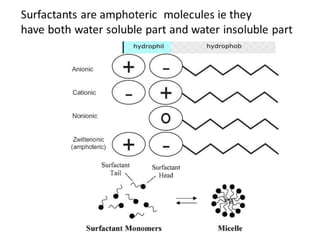
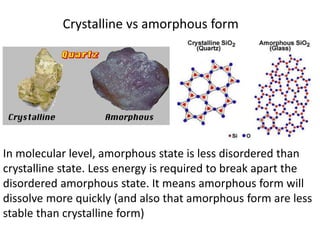
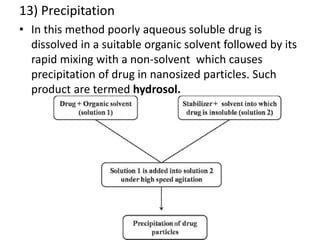
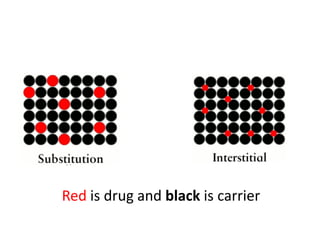
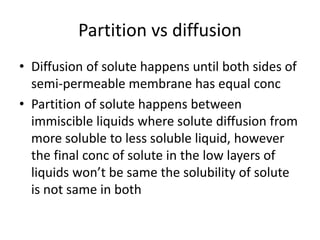
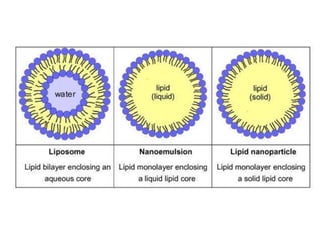
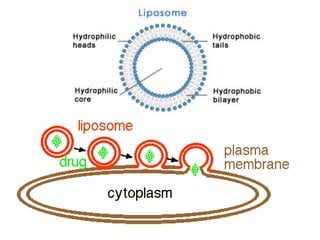
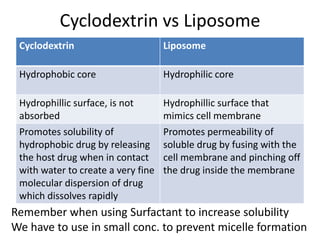
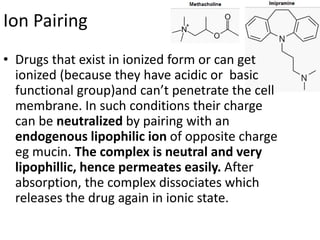
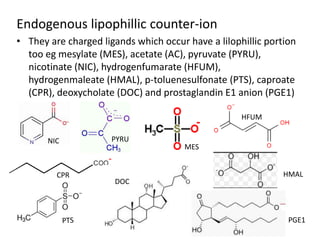
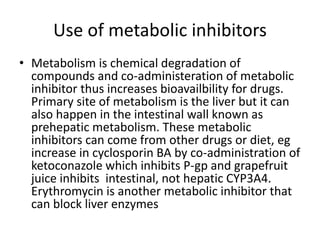
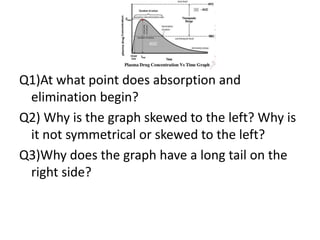
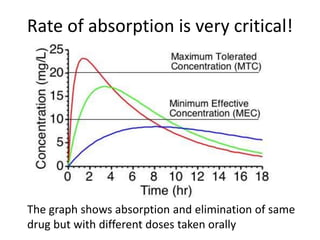
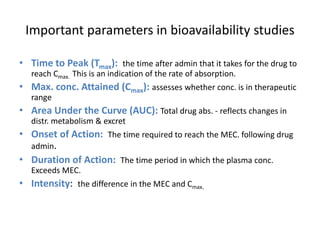
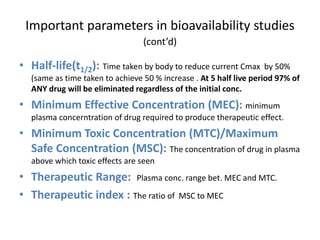
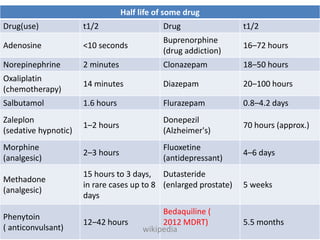
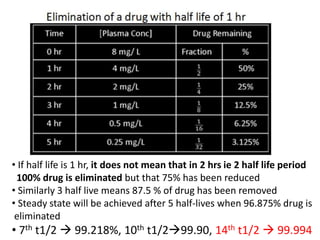
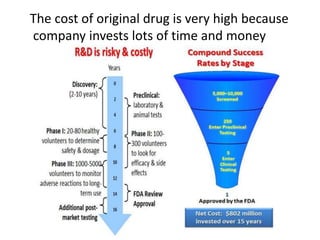
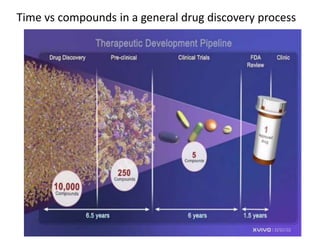
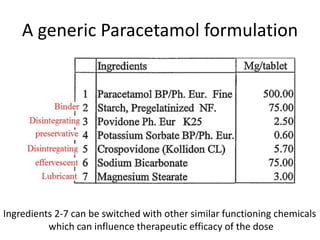
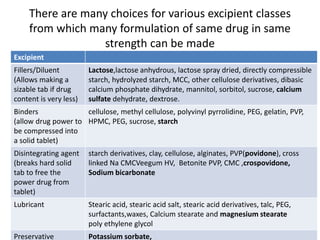
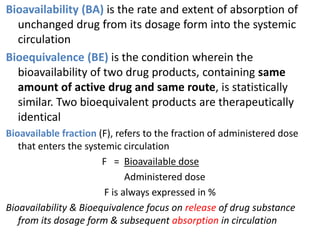
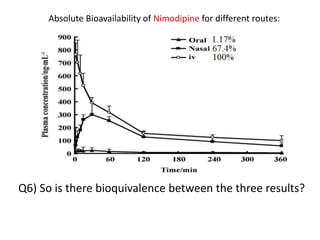
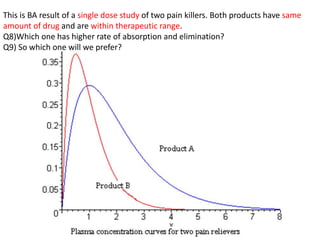
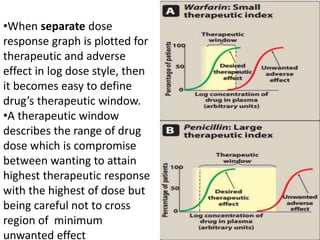
The document discusses pharmacokinetics and bioavailability, addressing questions around drug absorption, elimination, and the impact of different formulations. It outlines important parameters in bioavailability studies, such as time to peak (tmax), maximum concentration (cmax), and therapeutic range, highlighting the differences between innovator and generic drugs as well as their respective costs and efficacy. The document further explains methodologies for studying drug bioavailability and the considerations needed for clinical trials while emphasizing the role of various factors like excipients and patient physiology.





























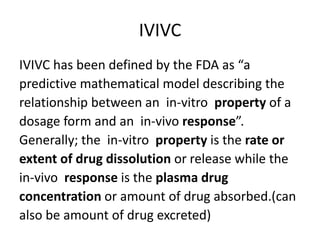
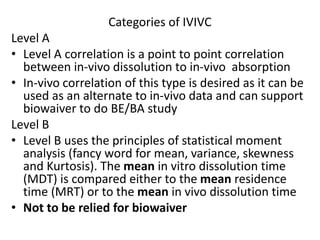
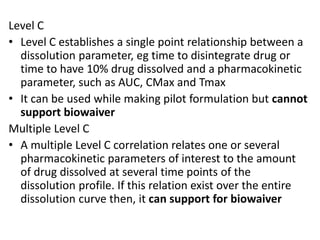
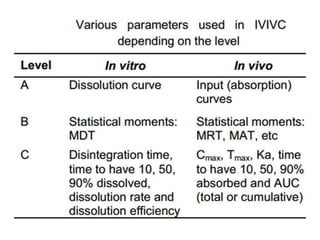
![Up to now summary
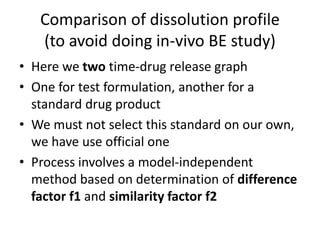
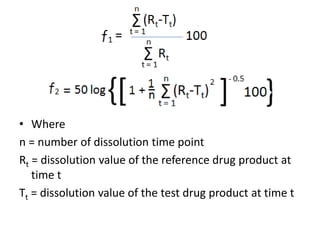
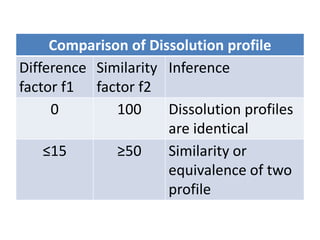
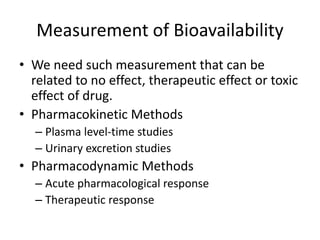
We are studying BA because:
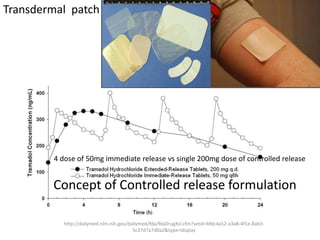
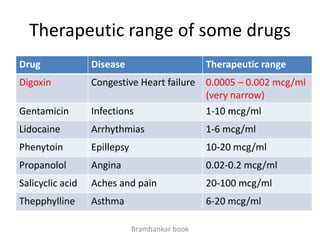
• to evaluate effect of formulation on BA to avoid cases like phenytoin
toxicity where we came to understand that change in formulation can
place BA outside the therapeutic range
• BA data is needed to do BE, ie to compare generic paracetamol (Niko) and
proprietary paracetamol (tylenol)
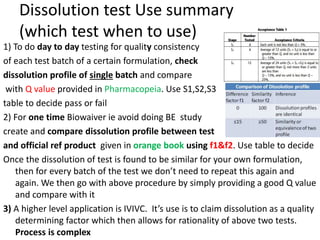
4 methods to do In-vivo (within living being) BA study.
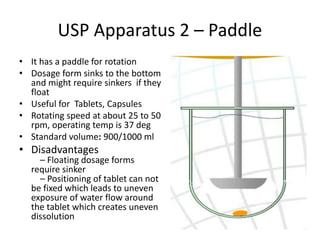
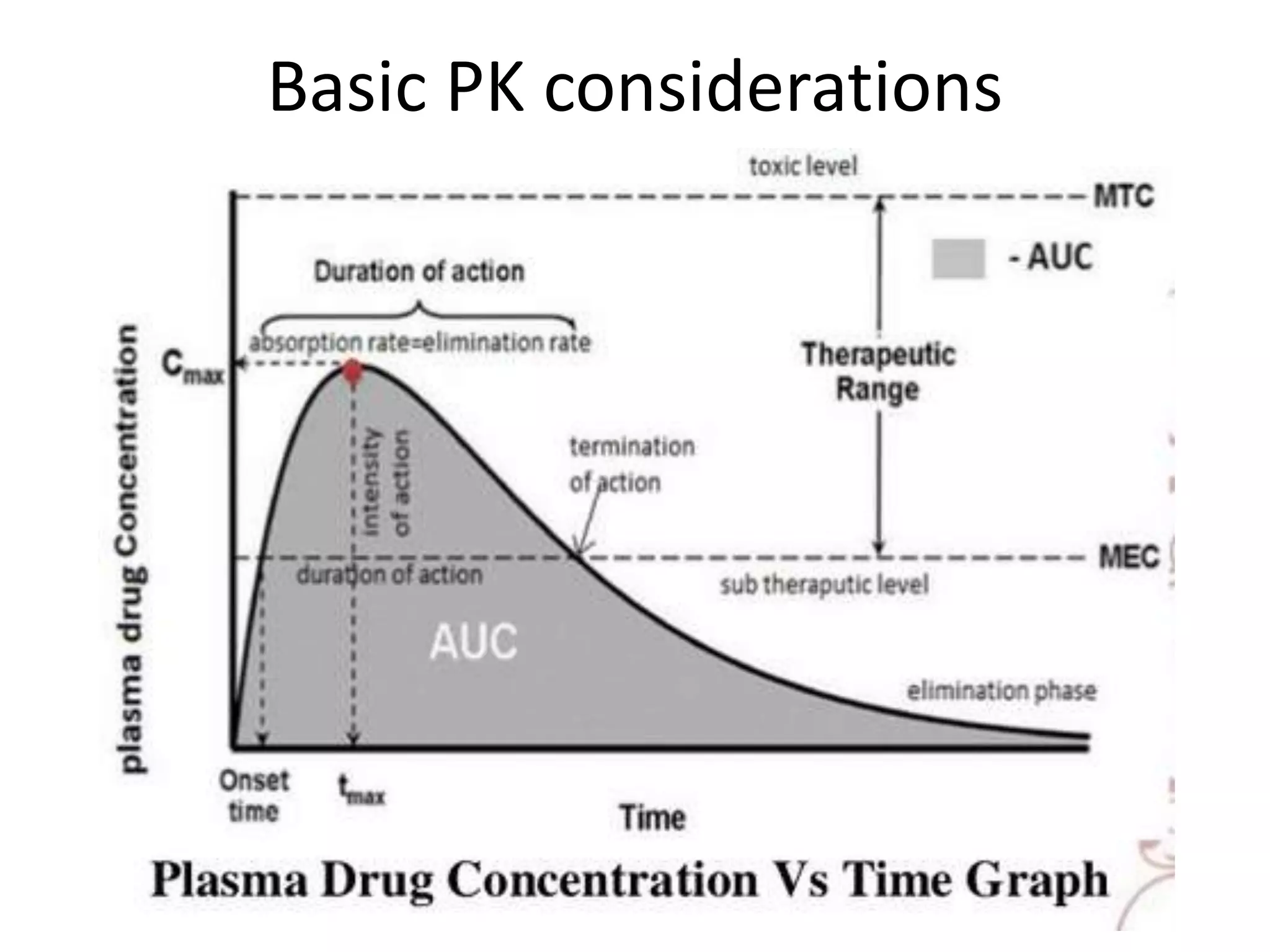
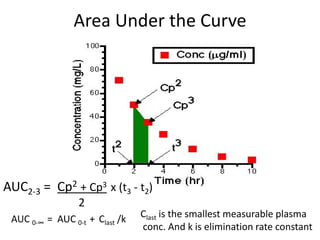
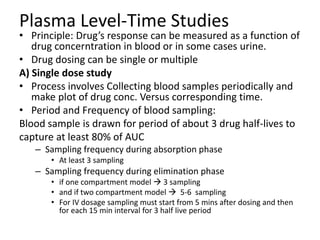
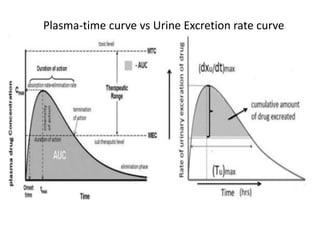
• Plasma – plasma drug conc vs time graph, method of choice
• Urine – plasma drug conc vs time graph, drugs that act on bladder
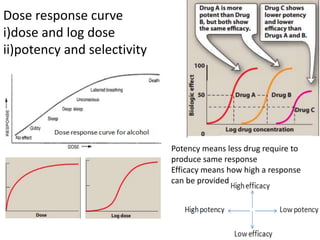
• Acute –dose/log[dose] vs response graph,
• Therapeutic - dose/log[dose] vs response graph, done on patients for
locally acting drugs such as antifungal cream, BE study problem
• For every method, three things to consider
– Volunteer type and size (healthy 20-40 aged male, same race, around 20-30
people)
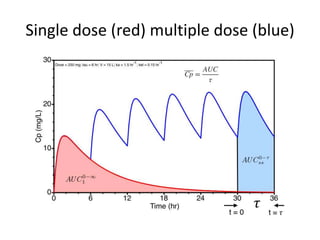
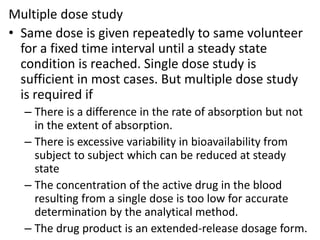
– Single or multiple dose ( single mostly preferred by FDA)
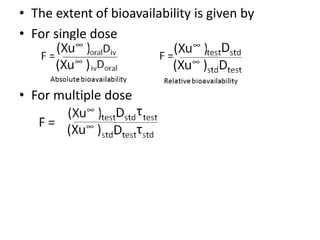
– Absolute or relative (absolute gives data about effect of 1st pass metabolism
and rate of absorption)](https://image.slidesharecdn.com/bioavailabilityandbioequivalence-150515181157-lva1-app6892/85/Bioavailability-and-bioequivalence-lecture-61-320.jpg)