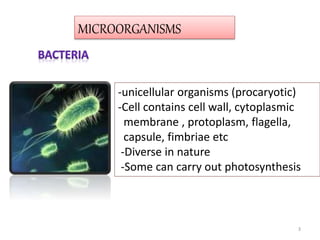
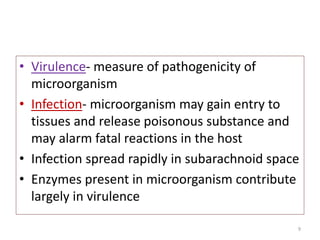
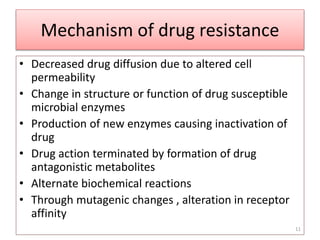
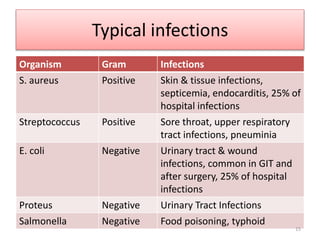
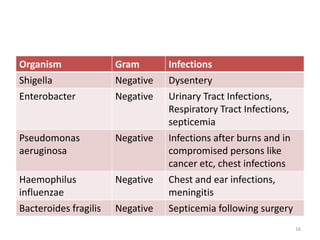
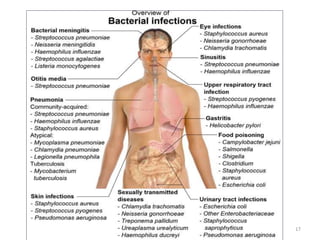
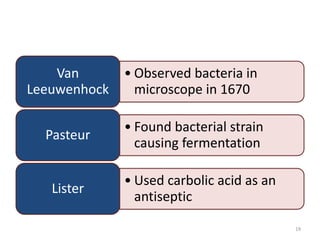
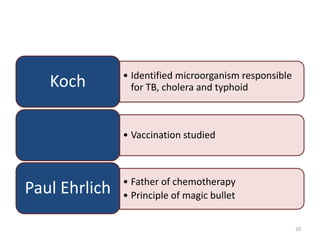
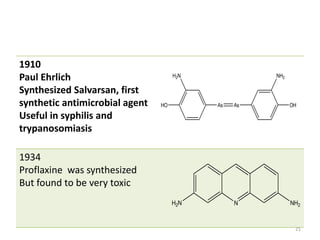
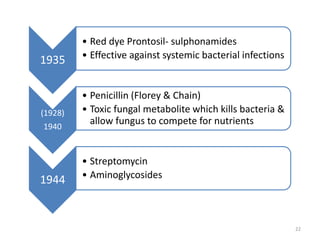
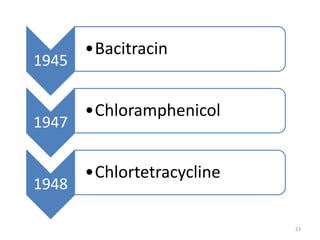
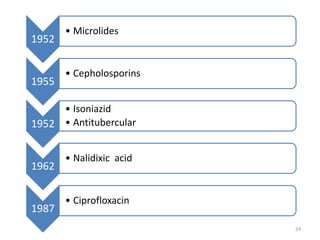
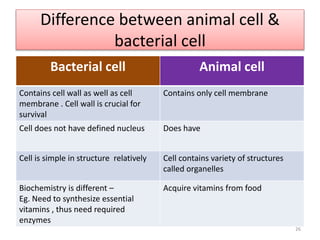
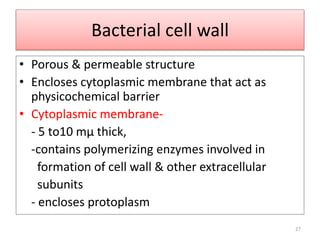
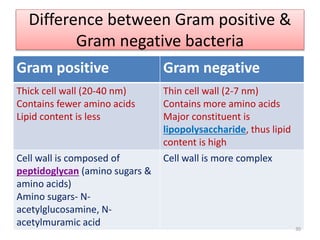
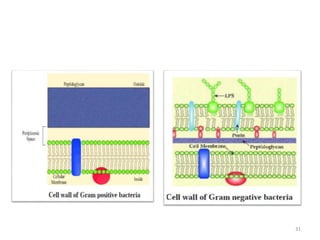
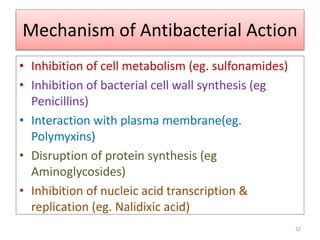
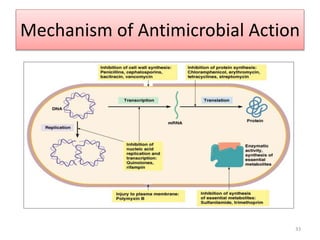
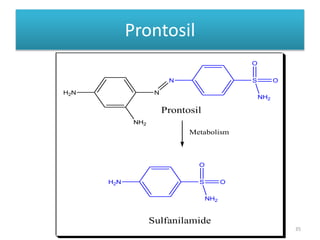
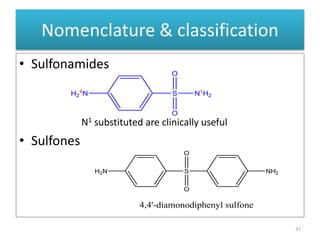
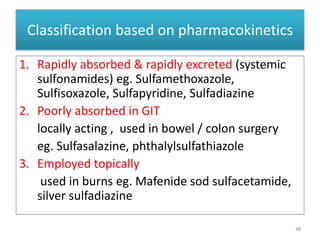
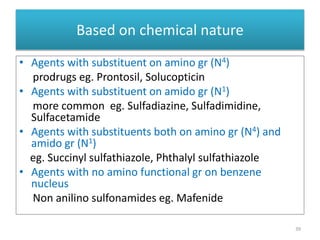
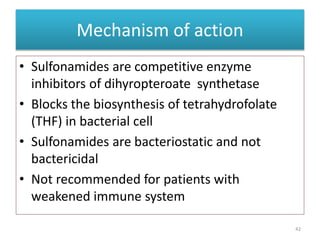
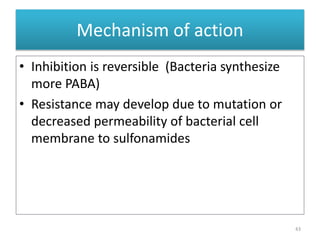
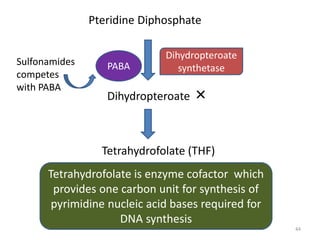
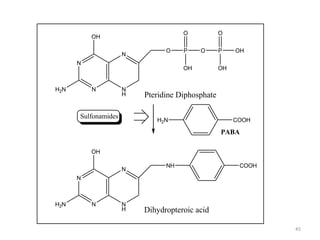
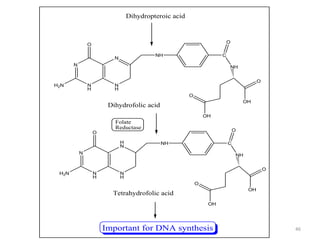
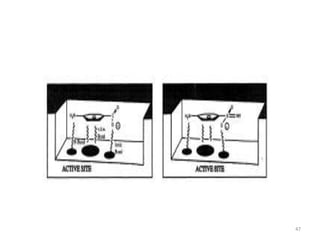
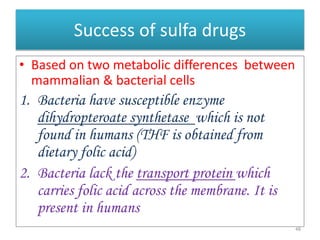
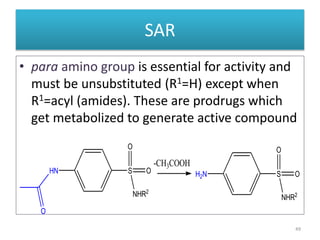
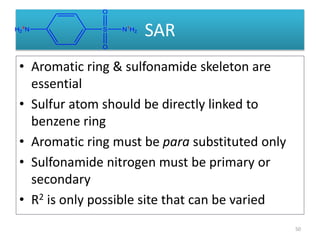
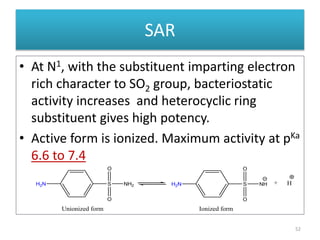
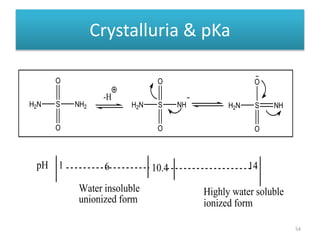
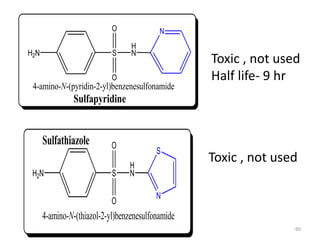
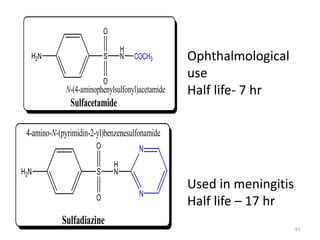
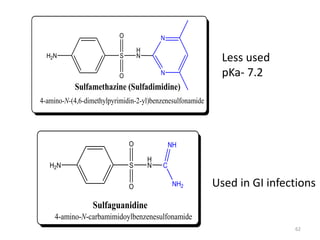
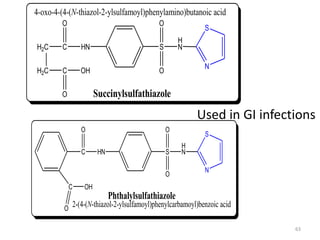
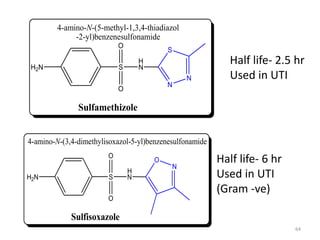
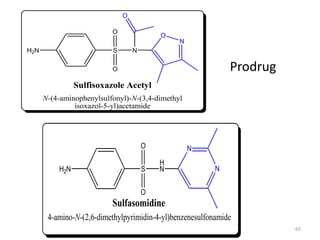
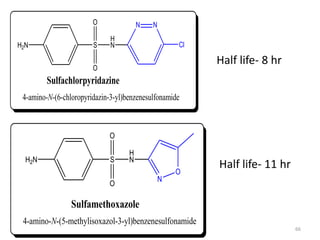
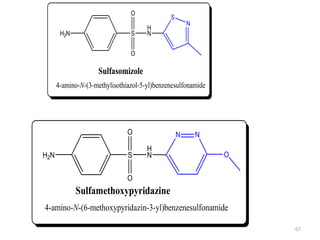
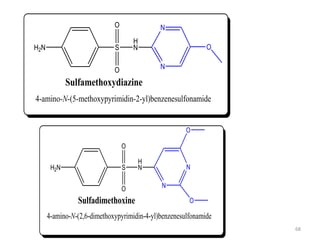
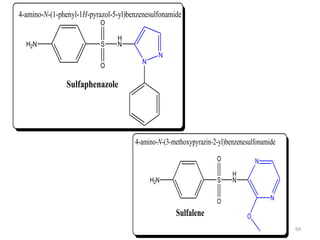
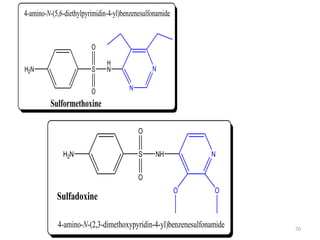
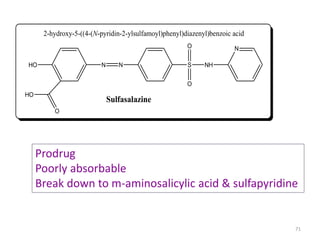
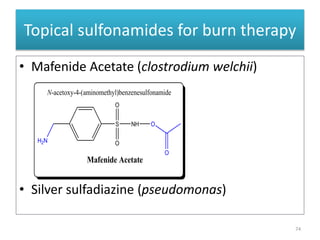
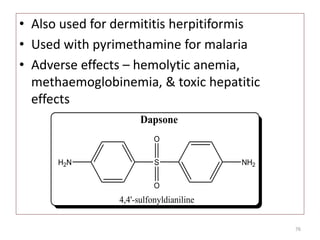
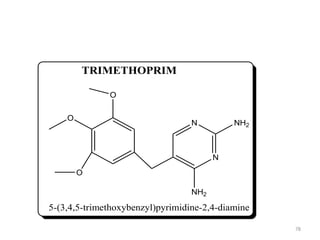
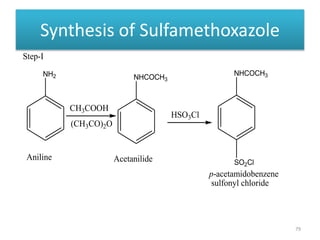
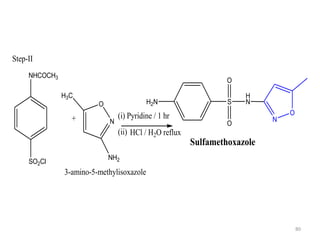
The document provides a comprehensive overview of sulfonamides, their mechanisms of action, drug resistance, and various applications in treating bacterial infections. It elaborates on the structural differences and pharmacokinetics of sulfonamide compounds, as well as their adverse effects and historical context in the development of antimicrobial therapy. Key highlights include the mechanisms of bacterial resistance and the classification of sulfonamides based on pharmacological activity and duration of action.