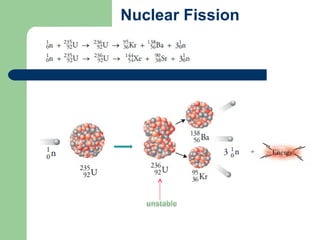
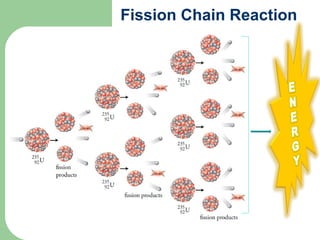
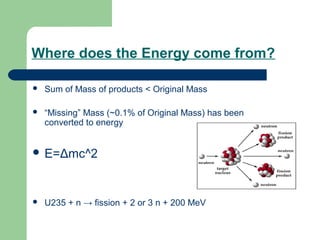
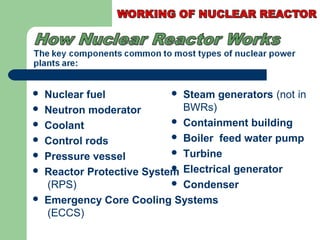
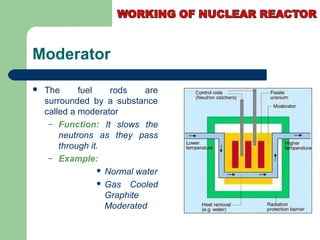
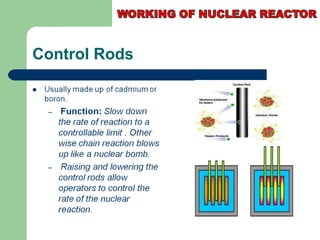
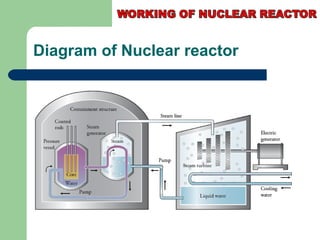
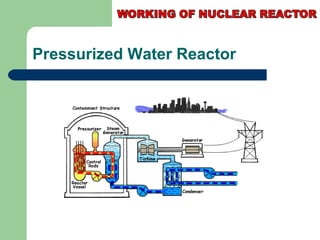
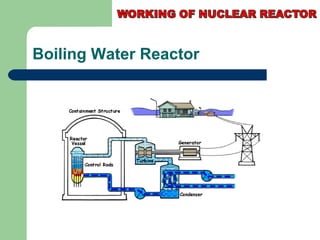
A nuclear reactor is a device that maintains a self-sustaining nuclear chain reaction to produce controlled nuclear fission. Nuclear reactors were first conceptualized in the 1930s and the first artificial reactor was built in 1942. There are two main types of reactors - research reactors designed to produce radiation beams and power reactors that produce heat primarily to drive power generators. A reactor contains nuclear fuel, a neutron moderator, and a coolant and uses control rods to regulate the fission rate.