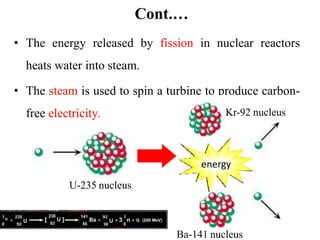
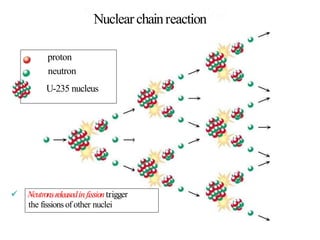
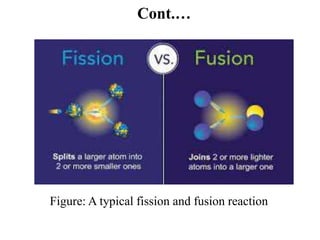
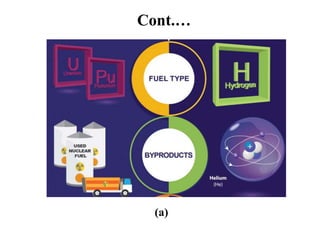
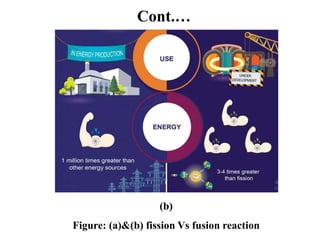
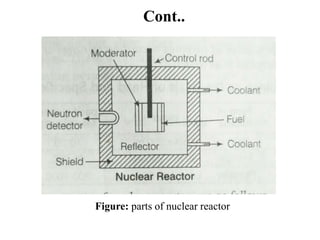
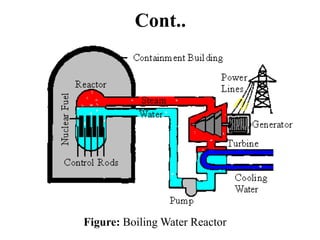
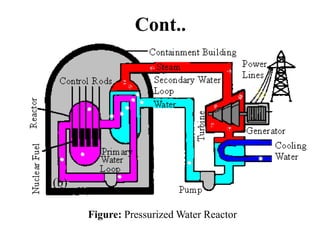
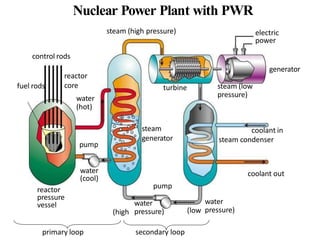
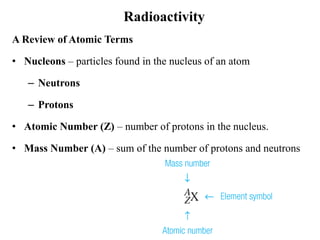
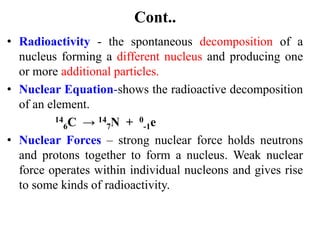
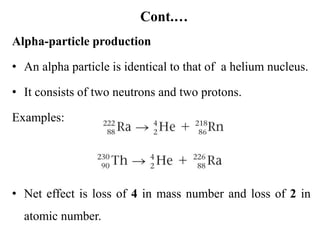
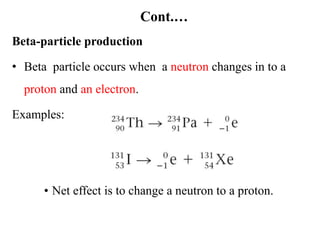
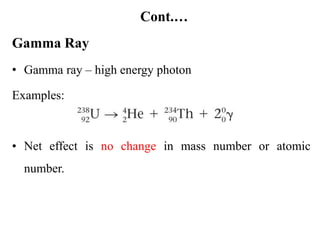
The document provides an overview of nuclear power plants, explaining the processes of nuclear fission and fusion that produce energy, primarily using uranium as fuel. It describes the components of a nuclear reactor, the types of reactors (boiling-water and pressurized-water), and the importance of controlling nuclear reactions for safety and efficiency. Additionally, it touches on radioactivity, types of radioactive decay, and applications of radioactive decay in medicine, science, and industry.