This document discusses the structure of atoms, including subatomic particles, isotopes, and physical states of matter. It also provides examples of chemical reactions and questions to test understanding of atomic structure concepts. Specifically, it defines atoms as composed of subatomic particles like protons, neutrons, and electrons. It discusses isotopes having the same number of protons but different numbers of neutrons. Examples are provided of physical changes of state when heating or cooling matter. Chemical reactions discussed include the reaction of ammonia and hydrochloric acid to form water vapor.
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
9. Complete the table below.
Isotope Uses
Carbon-14
Cobalt-60
Sodium-24
PAPER 2 : STRUCTURE
1.
(a) Diagram 1.1 shows the results of an experiment to investigate the movement of bromine particles in
air.
Air
Gar jar
Reddish brown
vapour spreads
Cover
throughout both
Bromine gas jars within 10
vapour minutes
Cover
Diagram 1.1
removed
(i) State the name of the process involved in this experiment.
………………………………………………………………………………………...…...………
[1 mark]
(ii) State the type of particles present in bromine gas.
………………………………………………………………………………………….....………
[1 mark]
(iii) Explain the observation in this experiment based on the kinetic theory of matter.
……………………………………………………………………………………….…...………
……………………………………………………………………………………….…...………
……………………………………………………………………………………….…...………
[3 marks]
2
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-2-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
Temperature
83
R T
Diagram 1.2
Time, s
(b) Diagram 1.2 shows a graph of temperature against time when solid X is heated?
(i) State the melting point of X.
………………………………………………………………………………...…………………
[1 mark]
(ii) What happens to the temperature while the substance changes state? Explain.
……………………………….…………………………………………………...…………..……
…………………………………………………………………………………...…………...……
…………………………………………………...………………………………………...………
[2 marks]
(iii) Explain the movement of particles X between R and T during heating.
……………………………………………………………………………………………...………
……………………………………………………………………………………………...………
[2 marks]
Glass wool soaked in concentrated Glass wool soaked in concentrated
ammonia solution hydrochloric acid
Diagram 2
2. Diagram 2 shows the set-up of apparatus to investigate the reaction between concentrated hydrochloric acid
and concentrated ammonia solution to form substance X. Based on the information, answer the following
questions.
(a) (i) What is the observation in the glass tube?
……………………………………………………………………………………...………
[1 mark]
3
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-3-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
(ii) Name substance X
……………………………..……………………………………………………………...………
[1 mark]
(iii) Name the process occurs in the glass tube.
…..………………………………………………………………………………………...………
[1 mark]
(b) Which gas diffused faster? Why?
…………………………..…………………………………………………………………...…….……
………….…….………………………………………………………………………………...……….
[2 marks]
(c) Write the chemical equation for the reaction in the experiment.
……………………..…………………………………………………………………………...………
[1 mark]
3. Table 3 shows proton number and nucleon number for atom W, X and Y.
Atom Proton number Nucleon number
W 11 23
X 17 35
Y 17 37
Table 3
(a) (i) What is meant by proton number?
.....................................................................................………………………………...............
[1 mark]
(ii) What is the number of neutron in atom W ?
......................................................................………………………………………................
[1 mark]
(b) Which atoms are isotopes? Explain why.
............................................................................................................................................................
...........................................................................................................................................................
[2 marks]
(c) Write the electron arrangement of atom X .
.......................... ...................................................................................................................................
[1mark]
4
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-4-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
(d) (i) Draw the electron arrangement of atom Y. In your diagram , show the number of
proton and the number of neutron in the nucleus.
[2 marks]
(ii) What is the number of valence electron of atom Y?
.....................................................................................................................................................
[1 mark]
(iii) Write the formula of ion Y.
.....................................................................................................................................................
[1 mark]
A
(e) Write the symbol of atom X in the form of Z X.
..............................................................................................................................................................
[1 mark]
4. (a) Diagram 4.1 shows the set-up of an experiment to determine the melting point of
solid X.
Thermometer
Water
Diagram 4.1
The temperature of X is recorded at 30 seconds intervals as shown below.
Time/second
0 30 60 90 120 150 180 210
Temperature/oC
70 77 80 80 80 82 85 95
(i) Plot the graph of temperature against time for the heating of X on the graph
paper.
[3 marks]
5
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-5-320.jpg)

![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
(ii) On the graph that you have drawn in (a), label the melting point of substance X.
[1 mark]
(iii) Explain why the temperature remains constant from 60 s to 120 s?
…………….……......................................................................................................................
…………..................................................................................................................................
[2 marks]
(iv) Draw the arrangement of particles in X at 85oC
[ 1 mark ]
(b) Why has the solid X in the boiling tube be stirred constantly with the thermometer during the
experiment?
……………………………....................................................................................................................
[1 mark]
(c) Why solid X is not directly heated without using water bath?
…………..……………….....................................................................................................................
[1 mark]
5. Table 5 shows four substances and their respective formulae.
Substance Chemical formula
Iodine I2
Copper Cu
Ethanol C2H5OH
Potassium chloride KCl
Table 5
(a) State 2 substances that consist of molecules.
…………………………….....................................................................................................................
[2 marks]
(b) Which of the following substances has a highest melting point?
……………………...............................................................................................................................
[1 mark]
(c) State the substance that can conduct electricity in the solid state.
……………………...............................................................................................................................
[1 mark]
(d) Name the particles present in potassium chloride.
……………………...............................................................................................................................
[1 mark]
7
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-7-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
PAPER 2 : ESSAY
6. Diagram 6.1 shows an atom of an element based on the model by James Chadwick.
Electron
Proton
Neutron
Diagram 6.1
(a) Compare the relative mass and the charge of two of the subatomic particles in diagram 6.1.
[4 marks]
7p
7n
Diagram 6.2
Diagram 6.2 shows an atom of element X.
(b)
(i) Describe fully the atomic structure shown in diagram 7.2.
[4 marks]
(ii) Write a symbol for the element in the form of
A
Z X
[2 marks]
(c) Graph 6.3 shows the heating curve of element Y.
Temperature /o C
Melting point
Graph 6.3
to t1 t2 t3 Time, minutes
Describe the graph in term of states of matter, particles arrangements and changes in energy.
[10 marks]
8
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-8-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
7 (a) What is meant by “melting point”?
During the melting of naphthalene, the temperature remains constant even though heat
is applied. Explain why.
[4 marks]
Condensation is the process where a gas changes to its liquid
state at a certain temperature and pressure when it is cooled.
(b) Describe the change of the kinetic energy, arrangement and the forces of attraction between the
particles at the following states:
(i) Before condensation
(ii) During condensation
(iii) After condensation
[10 marks]
(c) Diagram 7 shows the electron arrangement of ion Y3+.
ee 3+
e
e
e 14 n e n neutron
e e electron
e
ee Diagram 7
(i) Calculate the nucleon number of atom Y. [2 marks]
(ii) Y reacts with oxygen to form oxide Y, with the formula Y2O3.
The chemical equation for reaction Y with oxygen is show as:
4Y + 3O2 2Y2O3.
Given that the relative atomic mass of Y = 27 and O = 16.
Calculate the mass of oxide Y, Y2O3 formed when 10.8 g Y is completely burnt in
oxygen.
[4 marks]
PAPER 3 : STRUCTURE
Gas jar
cover Jelly
Water
Gas jar
Bromine Potassium
vapour manganate(VII)
A B C
Diagram 8
9
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-9-320.jpg)

![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
CHEMICAL FORMULA AND EQUATION
1. Write the chemical formula of the compound in the table below.
Ion Chloride Nitrate Hydroxide Sulphate Carbonate oxide
Sodium
Magnesium
Lead(II)
Copper (II)
Iron (II)
Iron (III)
Aluminium
2. Write balanced chemical equation for the following chemical reactions.
(a) Heat solid of copper (II) carbonate
………………………………………………………………………………………….
(b) Nitric acid reacts with sodium hydroxide
………………………………………………………………………………………….
(c) Hydrochloric acid reacts with zinc metal
………………………………………………………………………………………….
(d) Copper(II)nitrate reacts with magnesium
………………………………………………………………………………………….
(e) Chlorine gas reacts with lithium hydroxide
………………………………………………………………………………………….
(f) Hydrogen gas reacts with lead(II) oxide
………………………………………………………………………………………….
3. Avogadro constant, NA is defined as the number of particles in one mole of a substance
[1 Mol any substance consist of 6.02 X 1023 particles]. Calculate the number of particles in:
(a) 0.1 mol of calcium
(b) 1.5 mol of iron
(c) 2.0 mol of oxygen gas
(d) 1.5 mol of helium
(e) 2.0 mol hydrogen chloride
11
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-11-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
4. Calculate the number of moles of the following substances.
(a) 6.0 x 1023 aluminium
(b) 1.8 x 1021 argon
(c) 1.2 x 1023 bromine gas
(d) 2.4 x 1020 carbon dioxide
(e) 3.0 x 1023 ammonia
5. Calculate the mass of following substances.
a) 1.5 mol of chlorine gas b) 2.5 moles of oxygen gas
c) 2.0 moles of sulphuric acid d) 0.5 moles of ammonia, NH3
e) 2.5 moles of lead (II) carbonate f) 0.5 moles copper(II) nitrate, Cu(NO3)2
Molar volume: The volume of one mole of the gas
[22.4 dm3 mol-1 at STP, standard temperature and pressure]
[24 dm3 mol-1 at Room condition]
6. Calculate the volume of gases below.
(a) 0.5 mol of chlorine at STP.
(b) 0.2 mol of carbon dioxide at Room condition.
(c) 1.5 mol of methane at room condition.
(d) 0.5 mol of helium at room condition.
(e) 2.5 mol of ammonia at STP
12
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-12-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
7. Calculate the number of moles of the gases below.
(a) 250 cm3 of carbon dioxide in room temperature.
(b) 500 cm3 of hydrogen sulfide at STP.
(c) 200 cm3 of hydrogen chloride in room temperature.
(d) 750 cm3 of neon in room temperature.
(e) 300 cm3of ammonia at STP.
8. Magnesium powder reacts with hydrochloric acid to produce salt and hydrogen gas.
(a) Write the balance chemical equation for this reaction.
………………………………………………………………………………………….
(b) If 2.4 g of magnesium powder is added into excess hydrochloric acid, calculate,
(i) The mass of salt formed.
(ii) The volume of hydrogen gas liberated at room temperature.
9. Lead is extracted according to the following equation.
C + PbO CO2 + Pb
(a) Write the balanced chemical equation for the reaction.
(b) Determine the number of moles of lead extracted from 0.5 mole of lead (II) oxide.
(c) Calculate the number of moles of carbon required to extracts 0.5 mole of
lead(II) oxide.
(d) What is the mass of lead are produced if 44.6 g of lead (II) oxide is heated with
excess carbon.
[ RAM : Pb = 207, O = 16, C = 12 ]
13
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-13-320.jpg)



![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
PAPER 2: STRUCTURE
1. Diagram 1 shows the set-up of the apparatus for an experiment to determine the
empirical formula of an oxide of copper.
Oxide of copper
Dry hydrogen
gas
Heat
Diagram 1
Table 1 shows the results of an experiment after heating, cooling and weighing are
repeated until a constant weight is obtained.
Substance Mass(g)
Mass of combustion tube + porcelain dish 18.75 g
Mass of combustion tube + porcelain dish + oxide of copper 20.75 g
Mass of combustion tube + porcelain dish + copper 20.35 g
Table 1
(a) What is meant by empirical formula?
………..………………………………………………………………………………………….
[1 mark]
(b) Based on Table 5 results,
(i) Calculate the mass of copper and the mass of oxygen used in the experiment.
[2 marks]
(ii) Calculate the mole ratio of copper atoms to oxygen atoms.
Given that the relative atomic mass of Cu, 64; O ,16.
[2 marks]
(iii) State the empirical formula of this oxide of copper.
……….………………………………………………………………………………………
[1 mark]
(iv) Write the chemical equation for the reaction in this experiment.
……………...………………………………………………………………………………
[1 mark]
17
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-17-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
(c) Describe the steps that should be taken to ensure that all the air in the combustion
tube has been expelled , before any heating is carried out.
…………………………..…………………………………………..…………………………
. ……………………………….………………………………………………………………
……………………………………...…………………………………………………………
[3 marks]
2. (a) Culculate the relative molecular or formula masses of the following substances.
(i) Ethanol, C2H5OH.
Given that relative atomic mass of H = 1, C = 12, O = 16.
[1 mark]
(ii) Zinc nitrate, Zn(NO3)2
Given that relative atomic mass of O = 16 , N = 14, Zn = 65 .
[1 mark]
(b) A closed glass bottle contains 4 mol molecules of oxygen, O2.
(i) What is the number of oxygen molecules in the bottle?
[1 mark]
(ii) How many oxygen atoms are there in the bottle?
[1 mark]
(c) Find the number of moles of atoms in a sample containing 9.03 × 1020 atoms of
copper.
[1 mark]
(d) Calculate the mass, in gram, of 3.5 moles of copper(II) carbonate, CuCO3.
Given that relative formula mass of CuCO3 = 124
[1 mark]
(e) When silver carbonate, Ag2CO3 is heated, it will decompose to produce silver
metal, carbon dioxide gas and oxygen gas as shown in the equation below.
2Ag2CO3(s) 4Ag(s) + 2CO2(g) + O2(g)
18
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-18-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
A student heats 8.28 g silver carbonate. Calculate the volume of carbon dioxide gas, CO2 collected
at room temperature.
Given that relative atomic mass of C = 12, O =16, Ag = 108,
[3 marks]
3. Diagram 3 shows 3.1 g of copper (II) carbonate being heated strongly in attest tube. The gas
given out is passed into limewater in a test tube through a delivery tube.
Copper(II)
carbonate
Heat
strongly
Lime water
Diagram 3
(a) State the observation made when copper(II)carbonate powder is heated until the
reaction is complete.
…………………………………………………………………………………………………
[1 mark]
(b) Write the chemical equation to represent the reaction that takes place.
……………………………………………….…………………………………………………
[1 mark]
(c) Calculate the number of moles of copper(II)oxide produced.
[2 marks]
(d) Calculate the volume of gas produced at STP.
[2 marks]
(e) (i) What can be observed if the product is heated in a stream of hydrogen gas?
..………………………………………………………………………………………
[1 mark]
(ii) Calculate the mass of the substance produced.
[2 marks]
19
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-19-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
PAPER 2 : ESSAY
4.
(a) The information below is regarding substance X
Carbon 85.70%
Hydrogen 14.30%
Relative molecular mass = 56
(i) Determine the empirical formula of substance X.
[Given that the relative atomic mass of C = 12 , H = 1]
(ii) Determine the molecular formula of substance X.
(iii) Based on the answers in (a)(i) and (a)(ii) , compare and
contrast the empirical formula and the molecular formula.
[ 8 marks]
(b) Magnesium can react actively with oxygen to form magnesium oxide.
Describe an activity that can be carried out in the laboratory to determine the empirical formula of
magnesium oxide.
Include the calculations involved in your answer.
Given that the relative atomic mass for O = 16, Mg = 24.
[11 marks]
20
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-20-320.jpg)
![Set 1 Structure of the atoms & Chemical Equation Perfect Score F4 2010
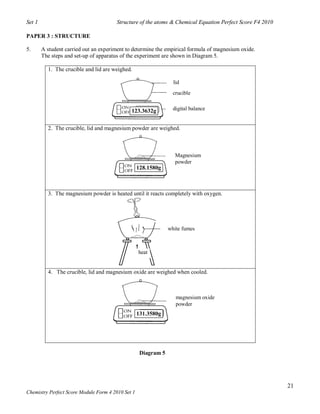
(a) Complete the following table by stating the observations and related inferences in
the experiment.
Observations Inferences
(i) ................................................ (i) ................................................................
.................................................... ....................................................................
(ii) ............................................... (ii) ...............................................................
.................................................... .....................................................................
(iii) ) .................................................... (iii)
.................... ...............................................
....................................................
....................................................................
[6 marks]
(b) Round off the reading to two decimal places and record it in the table below.
Description Mass / g
The crucible and lid.
The crucible, lid and magnesium powder.
The crucible, lid and magenesium oxide.
(c) (i) Calculate the mass of magnesium that has been used.
(ii) Calculate the mass of oxygen which reacted with magnesium.
(iii) Determine the empirical formula of magnesium oxide.
Use the information that the relative atomic mass, O = 16, Mg = 24
(d) The student wants to determine the empirical formula of lead(II) oxide. He used the steps and set-up
of apparatus as the experiment before. Predict whether the empirical formula of lead(II) oxide can be
determined. Explain your answer.
...............................................................................................................................................................
22
Chemistry Perfect Score Module Form 4 2010 Set 1](https://image.slidesharecdn.com/chemistry-perfect-score-module-form-4-set-1-110109205729-phpapp01/85/Chemistry-perfect-score-module-form-4-set-1-22-320.jpg)