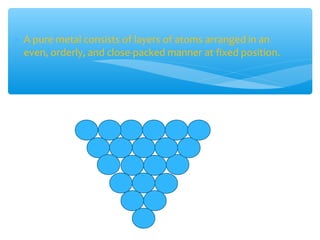
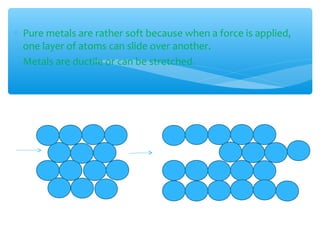
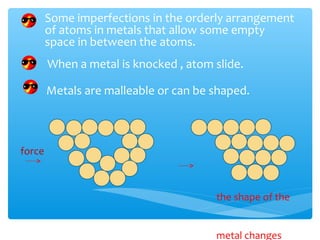
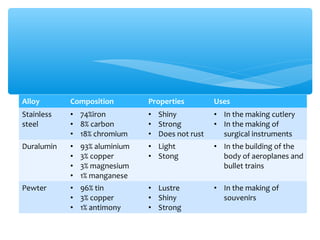
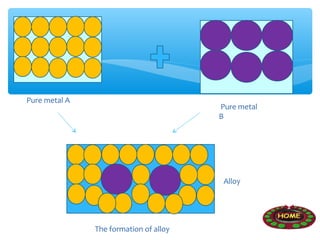
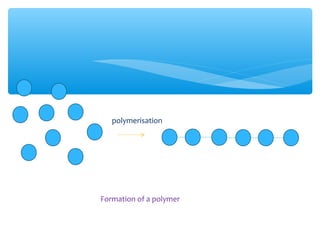
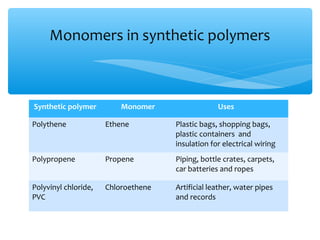
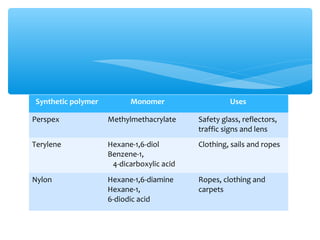
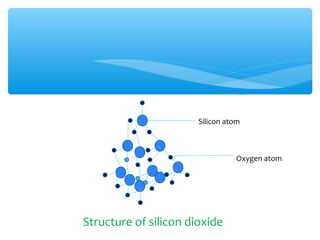
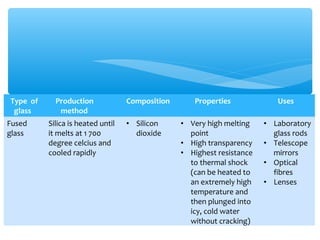
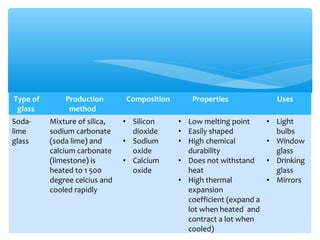
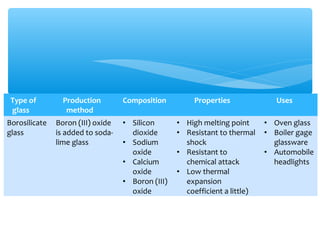
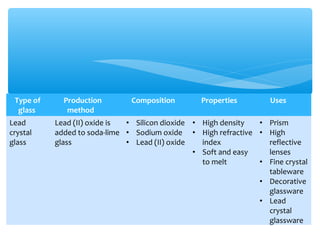
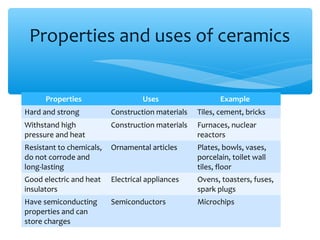
The document discusses several manufactured substances used in industry. It focuses on alloys, synthetic polymers, glass, and composite materials. For each category, it provides examples of specific materials, their compositions, properties, and common uses. Alloys discussed include bronze, brass, steel, stainless steel, and pewter. Synthetic polymers mentioned are polythene, polypropene, polyvinyl chloride, perspex, terylene, and nylon. The document also examines different types of glass and their characteristics.