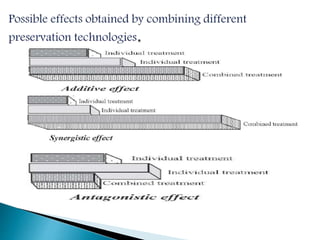
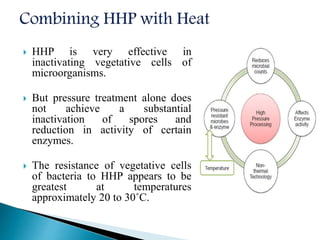
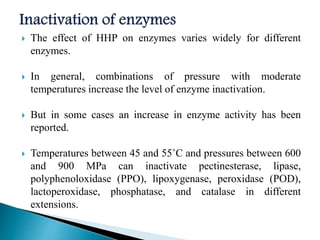
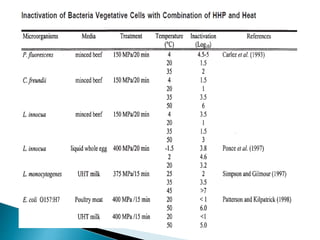
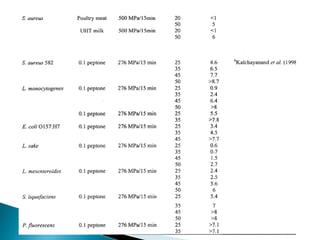
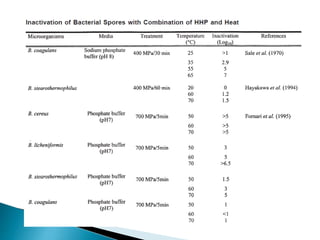
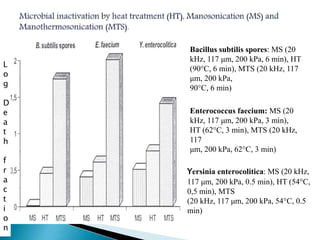
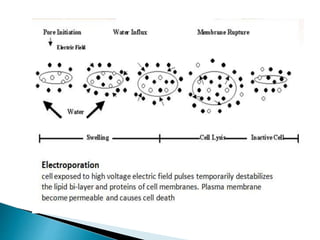
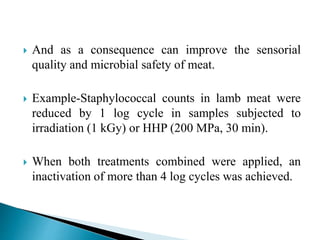
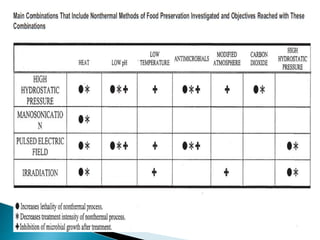
The document discusses various non-thermal preservation techniques for food, including combinations of high hydrostatic pressure (HHP), ultrasound, pulsed electric fields (PEF), and irradiation to effectively inactivate microorganisms while maintaining product quality. It highlights the benefits of using these combinations to enhance microbial inactivation, reduce treatment severity, and improve shelf-life and safety of food products. Future studies are suggested to further evaluate the commercial application of these combined technologies in the food industry.