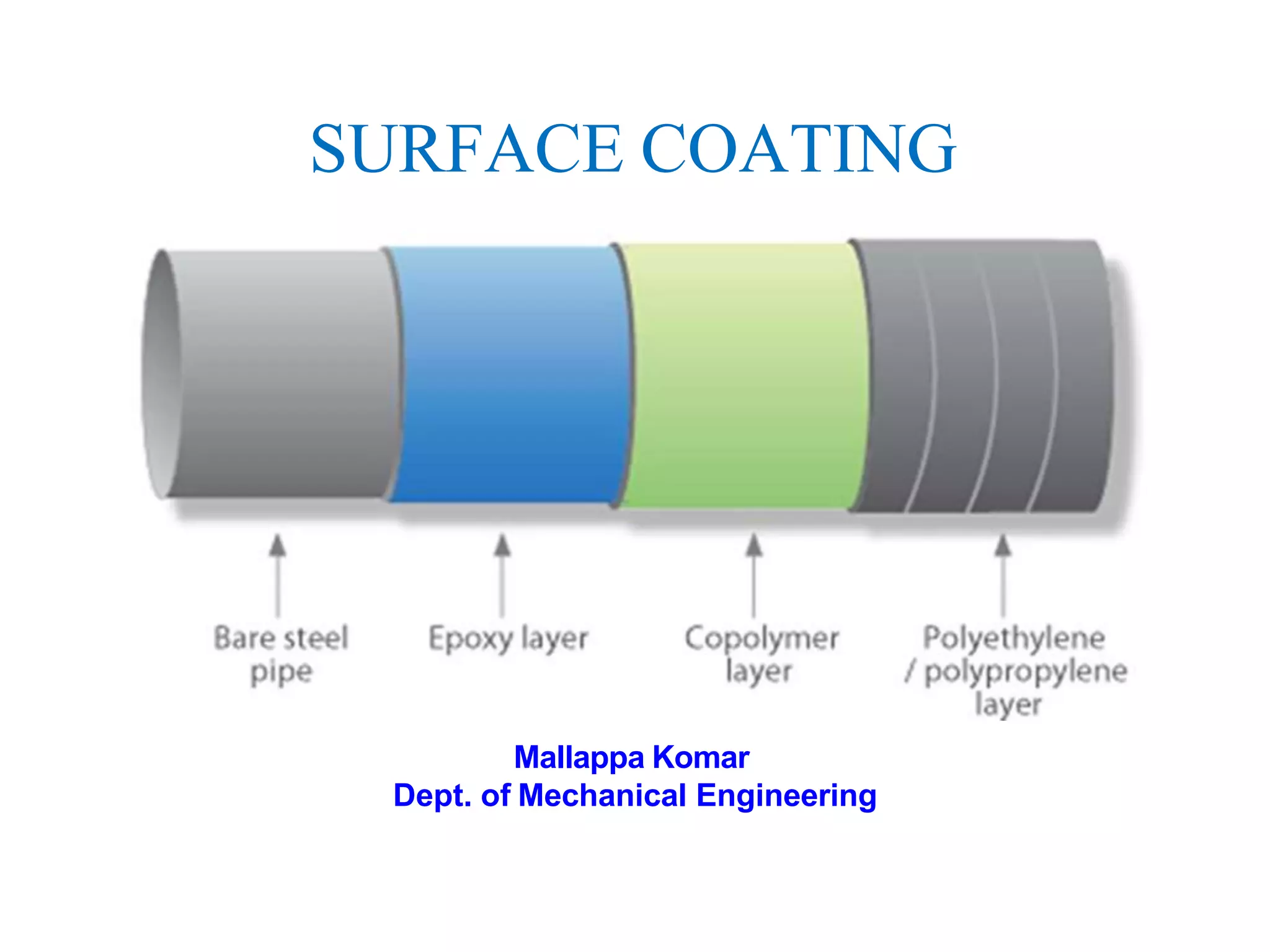
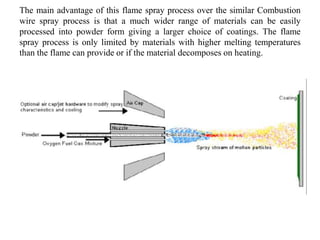
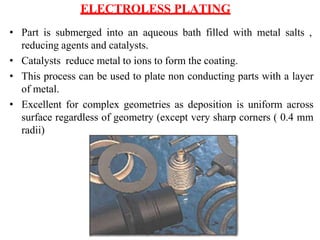
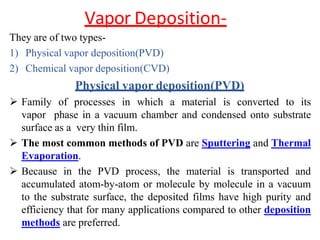
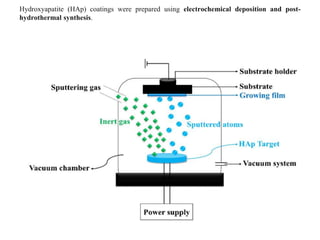
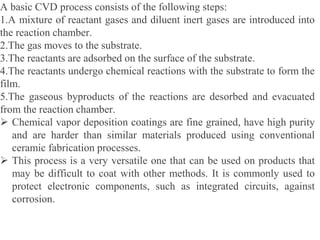
The document discusses surface coatings and corrosion. It defines corrosion as the deterioration of metals due to reaction with the environment. It then describes several types of coatings used to protect metals from corrosion, including conversion coatings like anodizing which form protective metal oxide layers, thermal coatings like flame spraying, electrochemical coatings like galvanization, and vapor deposition methods like PVD and CVD. The document emphasizes that coatings provide barrier protection and extend the lifetime of metal substrates.