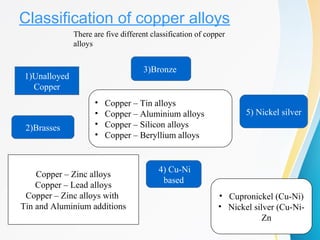
Copper and its alloys are discussed. Copper is extracted via both pyrometallurgical and hydrometallurgical methods from ores like chalcopyrite. Blister copper undergoes electrolytic refining to obtain pure copper. Copper alloys include brasses, bronzes, aluminum bronzes, beryllium bronzes and cupro-nickels. These alloys find applications in electrical, automotive and other industries due to copper's high thermal and electrical conductivity.