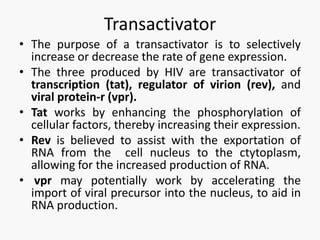
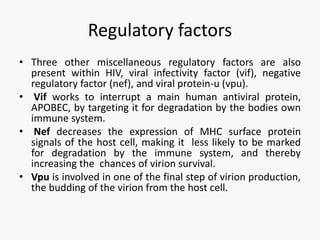
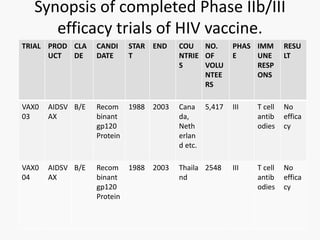
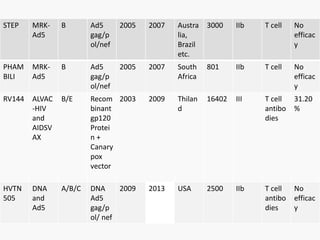
This document discusses the challenges involved in developing an HIV vaccine. It provides background on HIV, describing it as a retrovirus that targets CD4+ T cells. It reviews past vaccine trials, noting the only modest success of the RV144 trial. It discusses the massive diversity of HIV strains as a major challenge. It outlines various vaccine design strategies that have been pursued, including recombinant proteins, DNA vaccines, viral vectors, and approaches using broadly neutralizing antibodies. Throughout, it emphasizes the need for a vaccine to elicit robust cellular and humoral immune responses against a wide range of HIV subtypes to achieve protective efficacy.