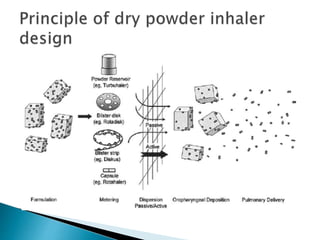
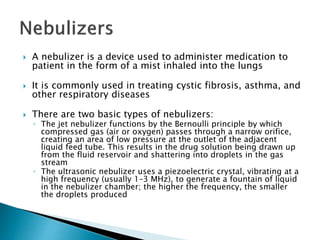
The document discusses pulmonary drug delivery systems including their anatomy, formulations, and devices. It describes the three main regions of the nasal cavity and different types of dosage forms that can be used for nasal drug delivery including drops, sprays, and gels. It also discusses dry powder inhalers, metered dose inhalers, and nebulizers as well as some advantages and disadvantages of pulmonary drug delivery. Key factors that influence nasal drug absorption and strategies to improve nasal drug delivery are also summarized.