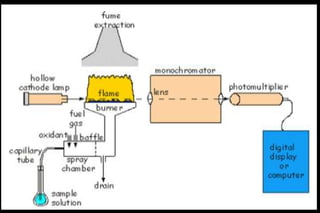
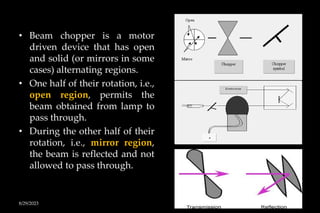
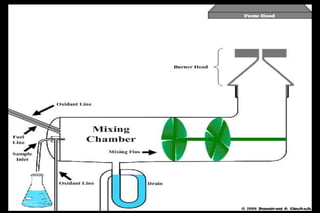
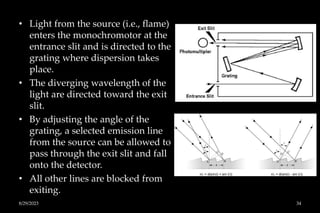
Atomic absorption spectrophotometry (AAS) is a technique used to determine the concentration of metals in solutions. It works by vaporizing the sample into atoms and measuring how much light of a specific wavelength is absorbed. The key components are a hollow cathode lamp, burner to create a flame, monochromator to select the wavelength, and detector. Standards of known concentration are used to generate a calibration curve to determine unknown sample concentrations from their absorbance readings. AAS can be used to analyze over 60 metal elements present in parts per million concentrations in various materials like water, foods, soils, and biological samples.