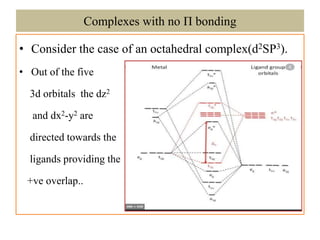
The document discusses the molecular orbital (MO) theory of coordination complexes. It explains that in MO theory, the symmetry of combining atomic orbitals must be the same to allow maximum orbital overlap. When forming molecular orbitals, lower energy atomic orbitals contribute more to bonding orbitals, while higher energy ones contribute more to antibonding orbitals. For an octahedral complex, the d-orbitals split into lower energy eg and higher energy t2g sets, and the document provides an example electronic configuration of the [Co(NH3)6]3+ complex.






![• The t2g orbitals are non bonding in a σ only system.
They are used for Π bonding.
• Electrons may be added to most of the complex in the
order of increasing energy.
• Consider the complex [Co(NH3)6]3+ .There will be a
total of 18 electrons, 12 from lone pairs on the
nitrogen atom and 6 from the 3d6 configuration of
Co3+ ion.
• Electronic configuration will then be a1g
2, t1u
6, e1g
4,
t2g
6 .The complex is diamagnetic.](https://image.slidesharecdn.com/motheory-200813052635/85/Mo-theory-8-320.jpg)