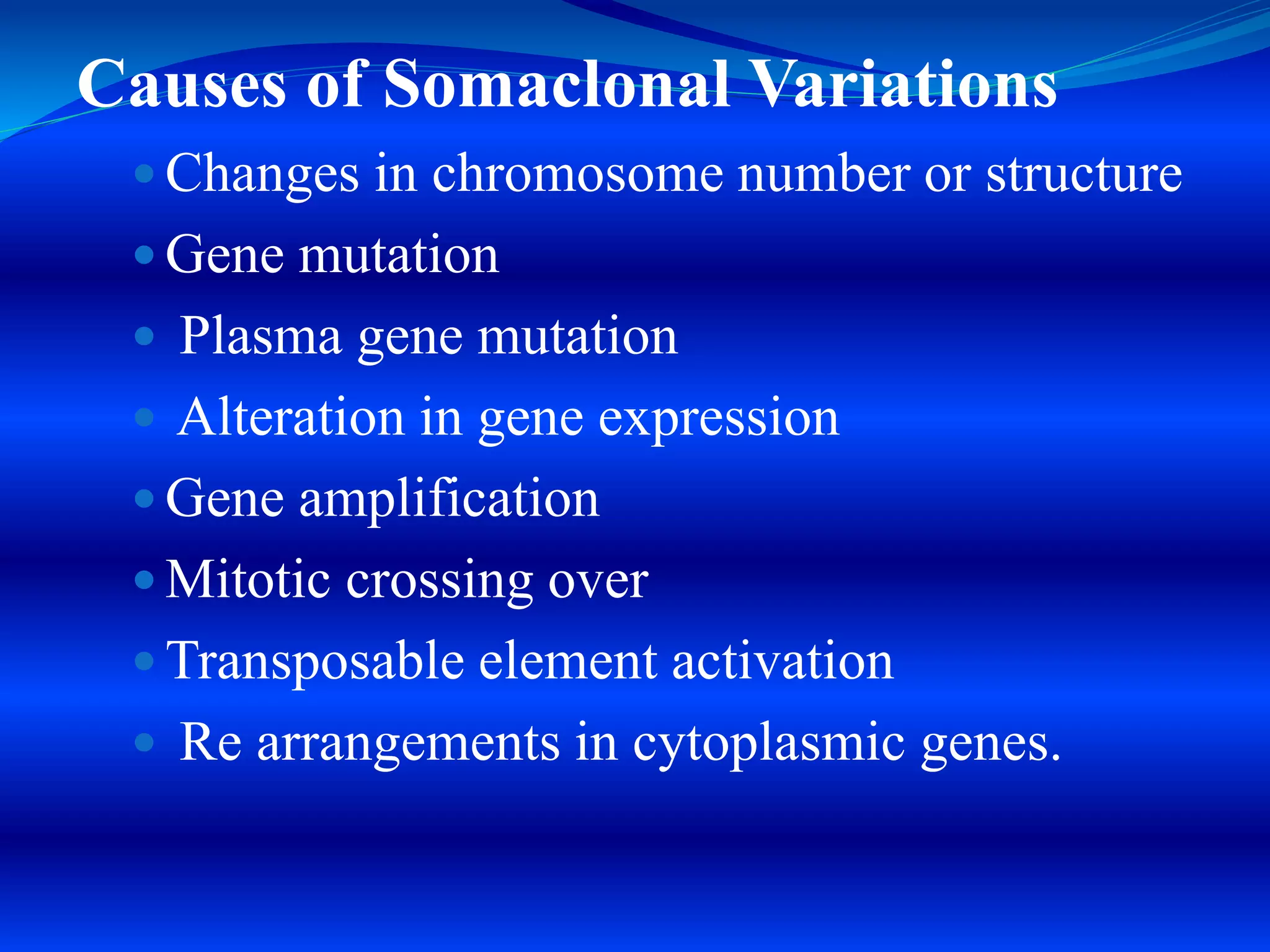
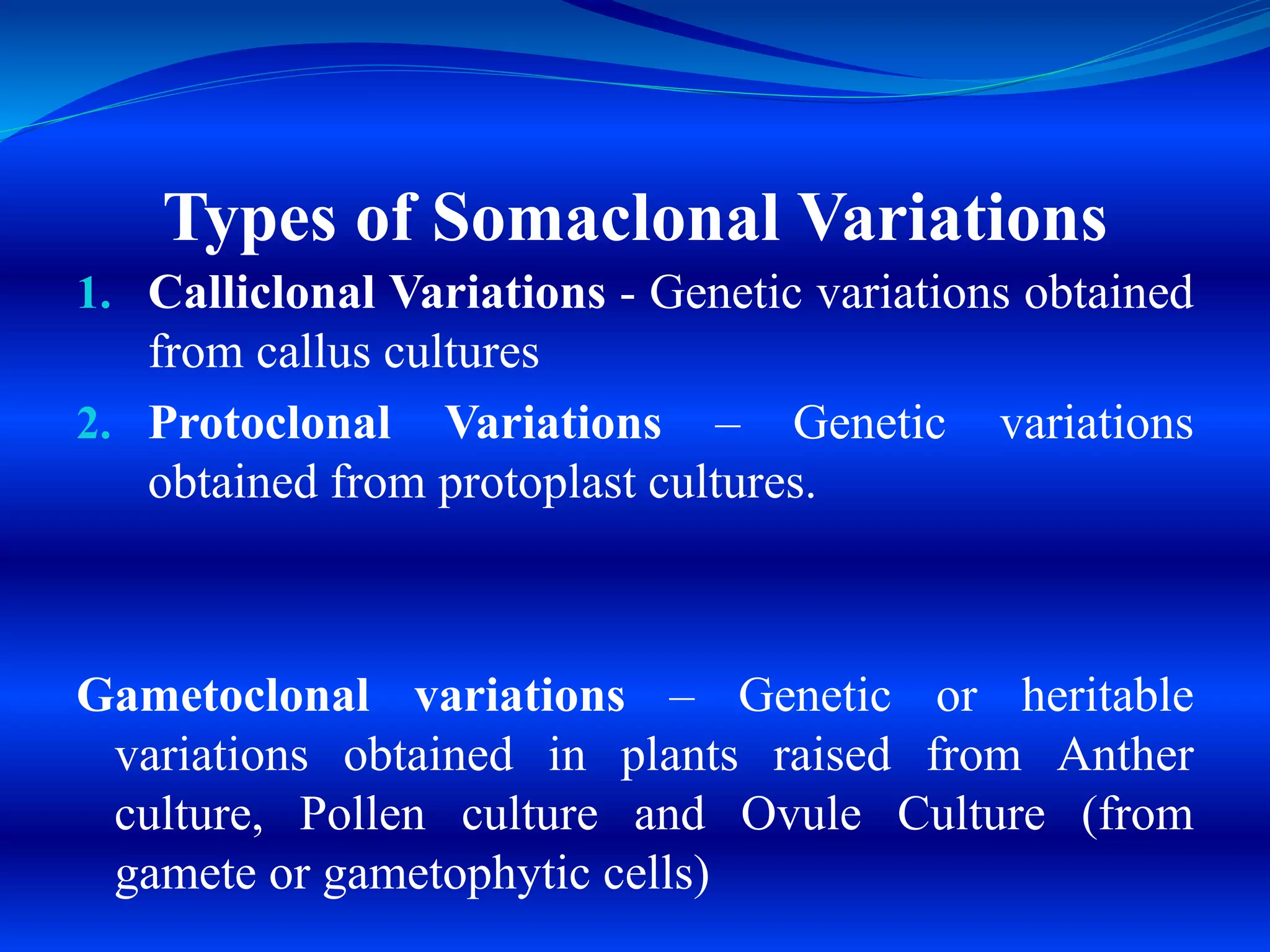
Micropropagation is a process used to rapidly multiply plant populations. It involves culturing small explants like shoot tips or leaf nodes on nutrient media to stimulate growth. Key methods include proliferation of axillary buds to generate shoots, and organogenesis to generate shoots or roots directly from cultured cells or tissues. Micropropagation is used commercially for many ornamental, fruit, plantation and endangered plant species. It provides advantages like high multiplication rates, production of virus-free plants, and ability to propagate plants quickly independent of seasons. However, it also carries risks like genetic variations in cloned populations.