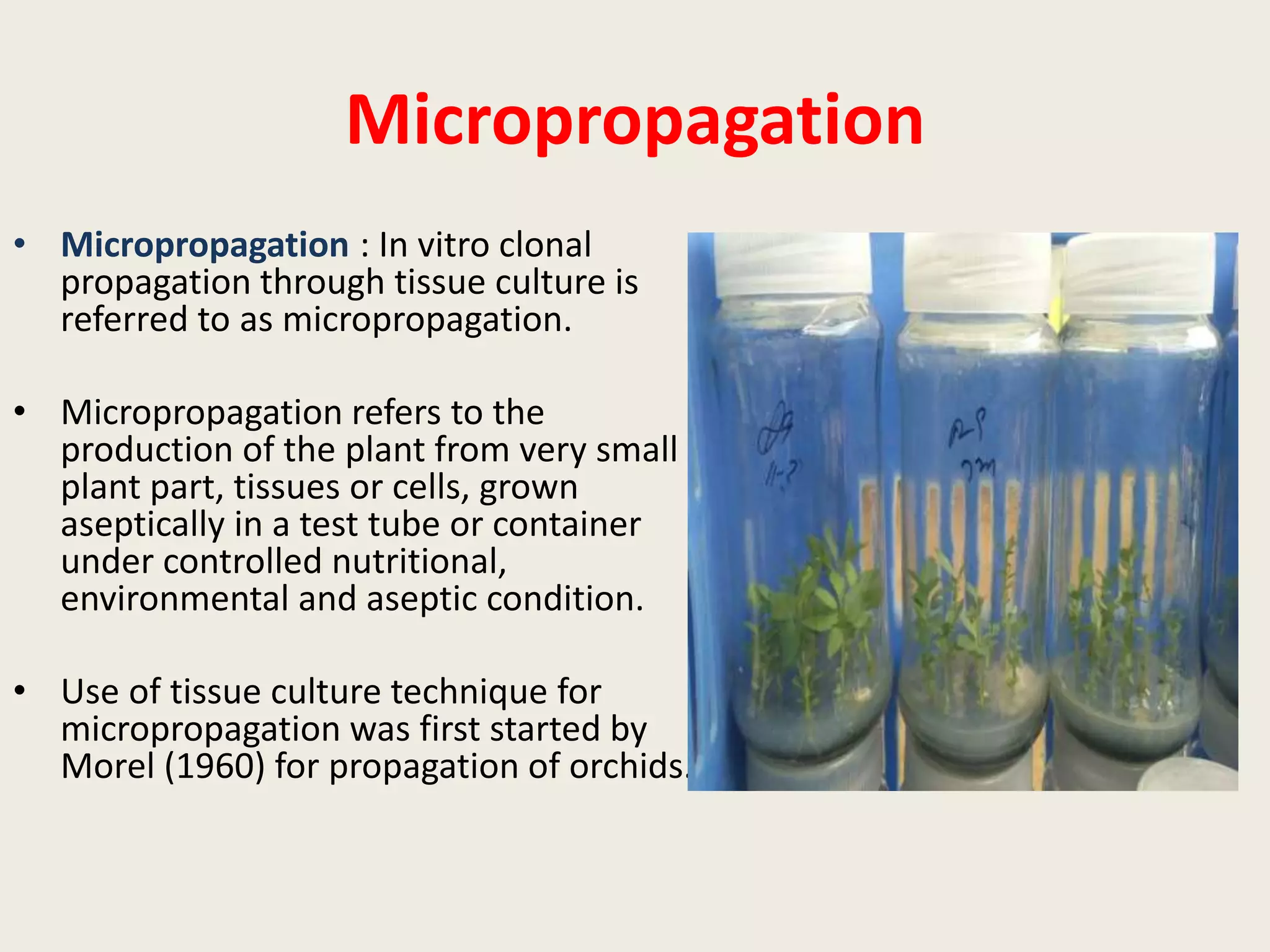
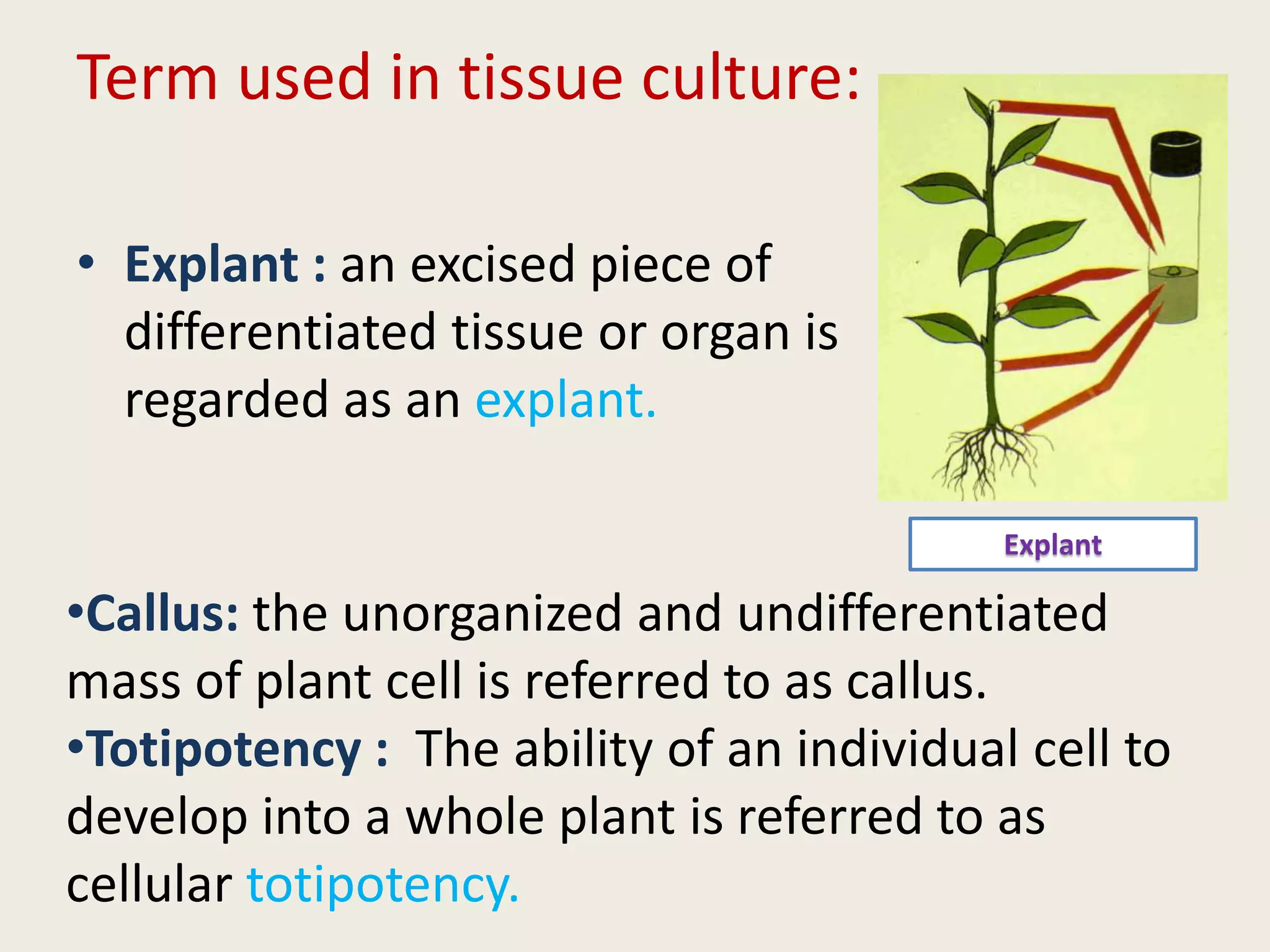
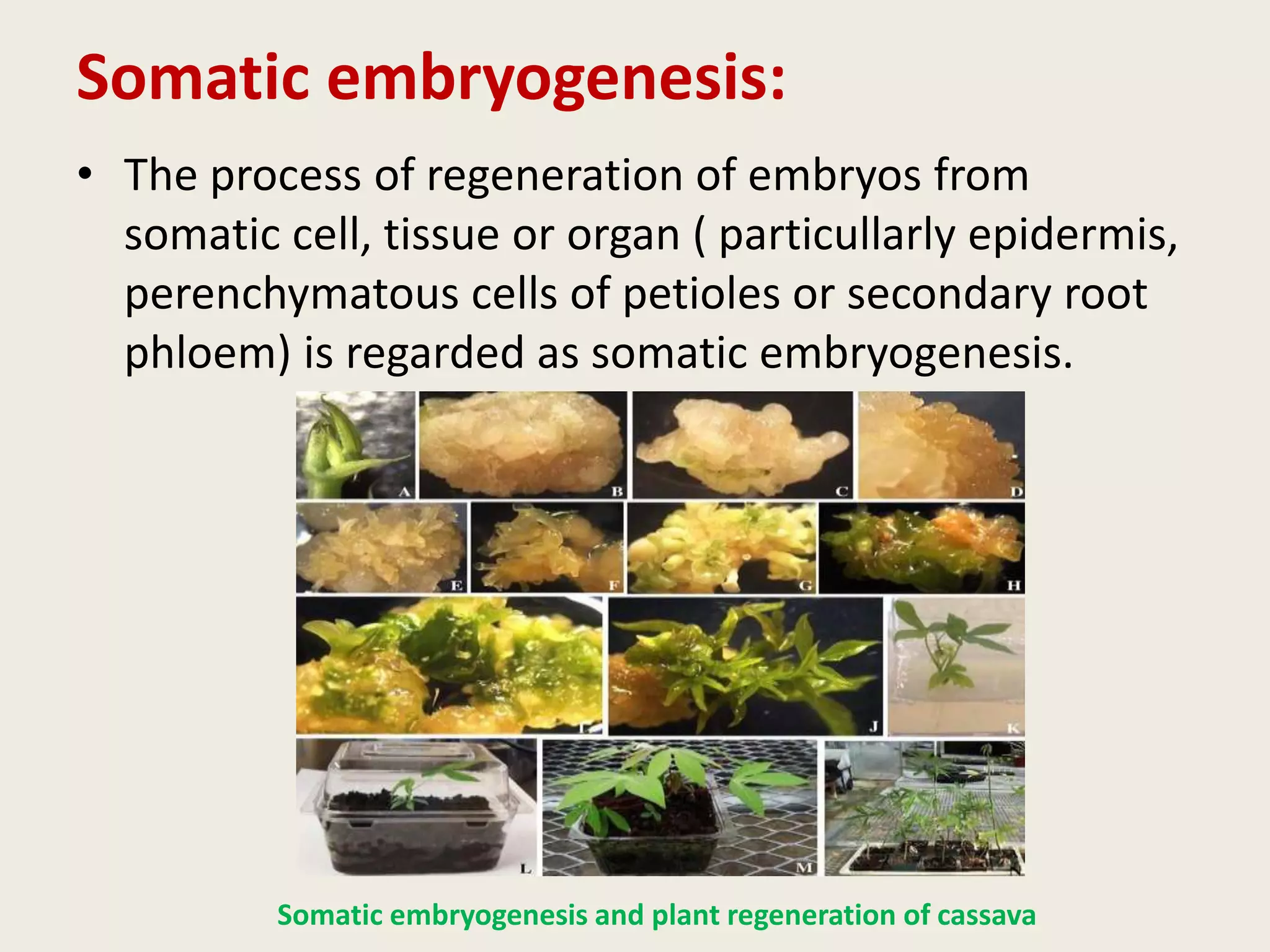
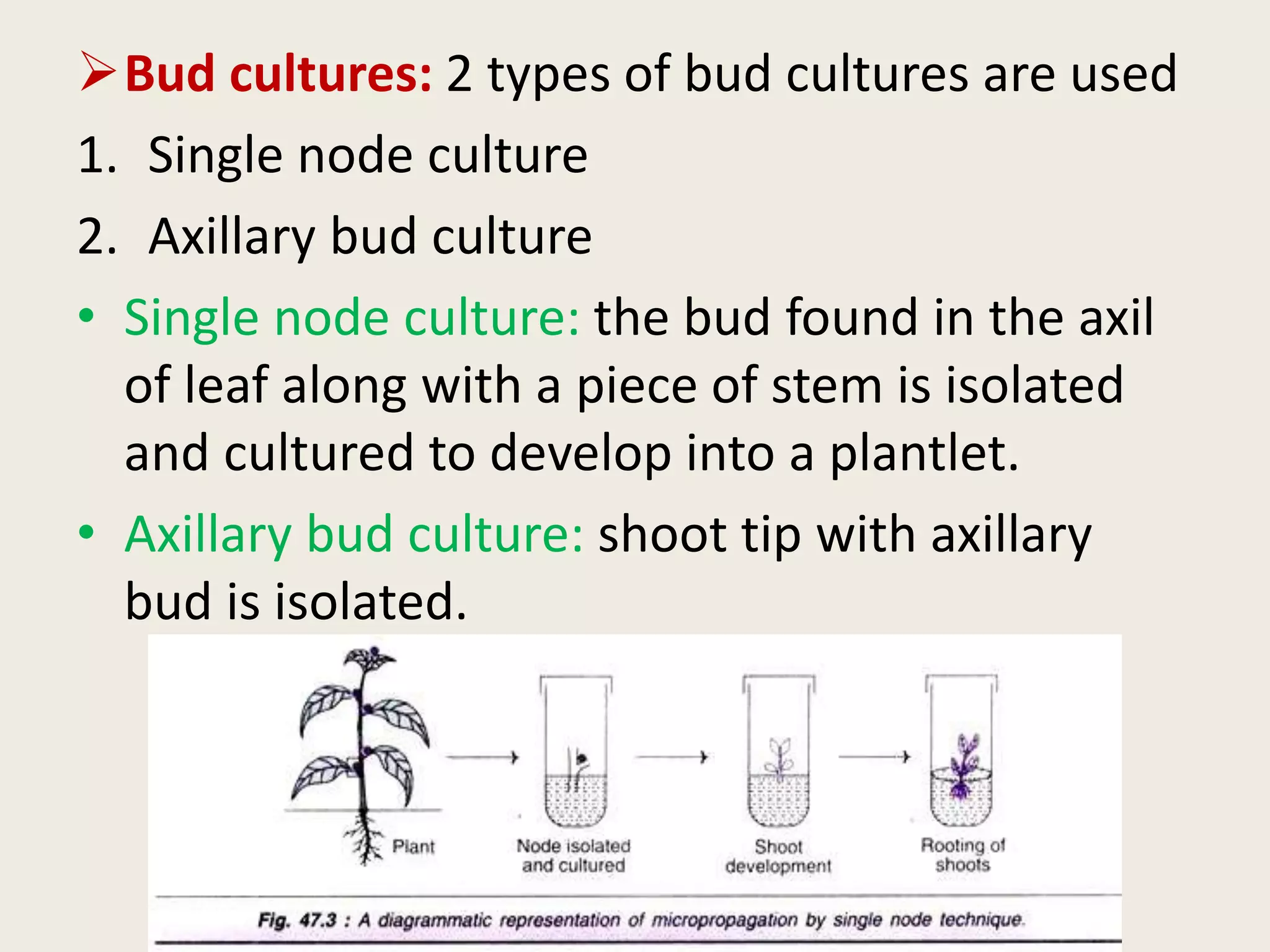
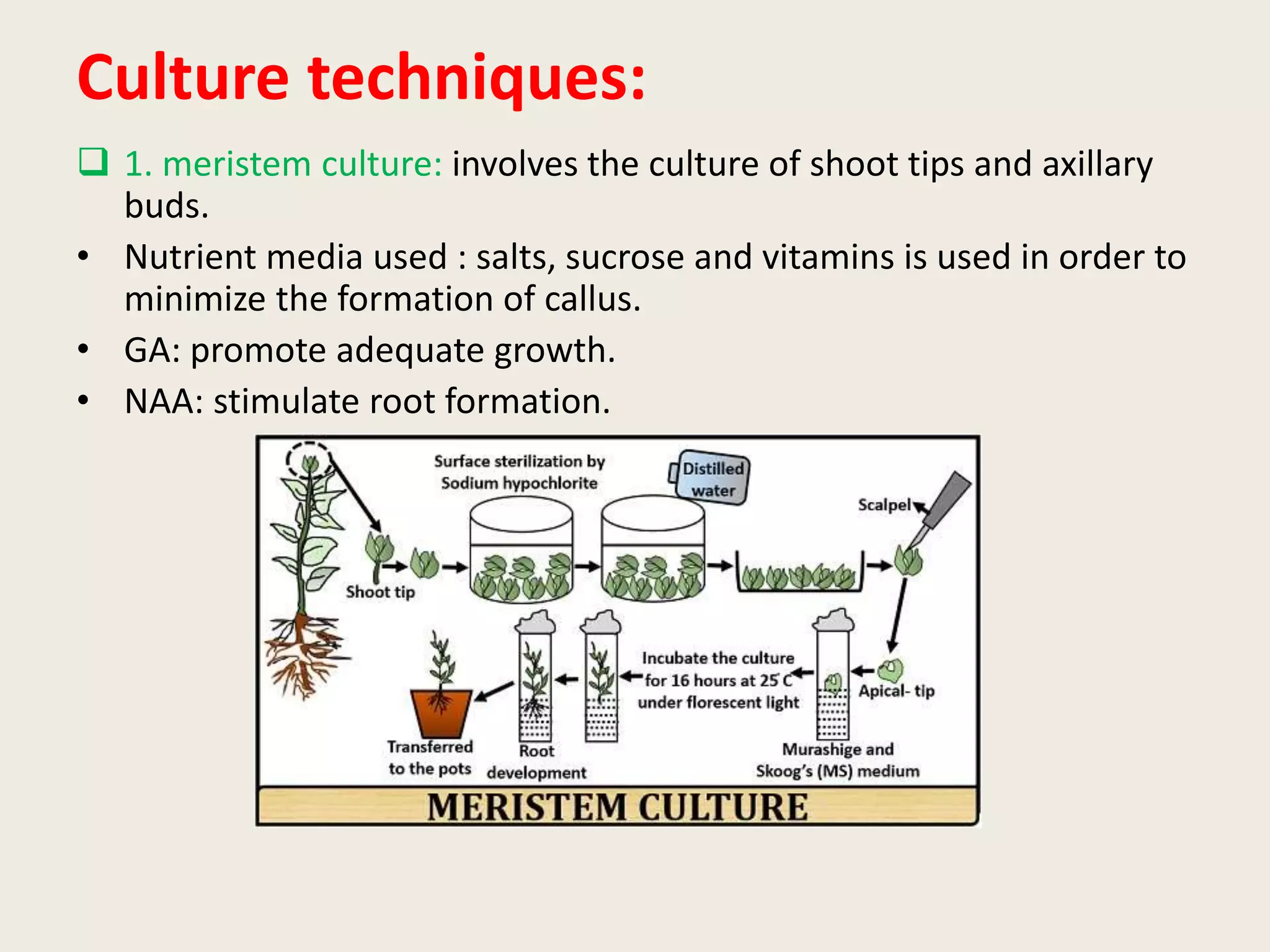
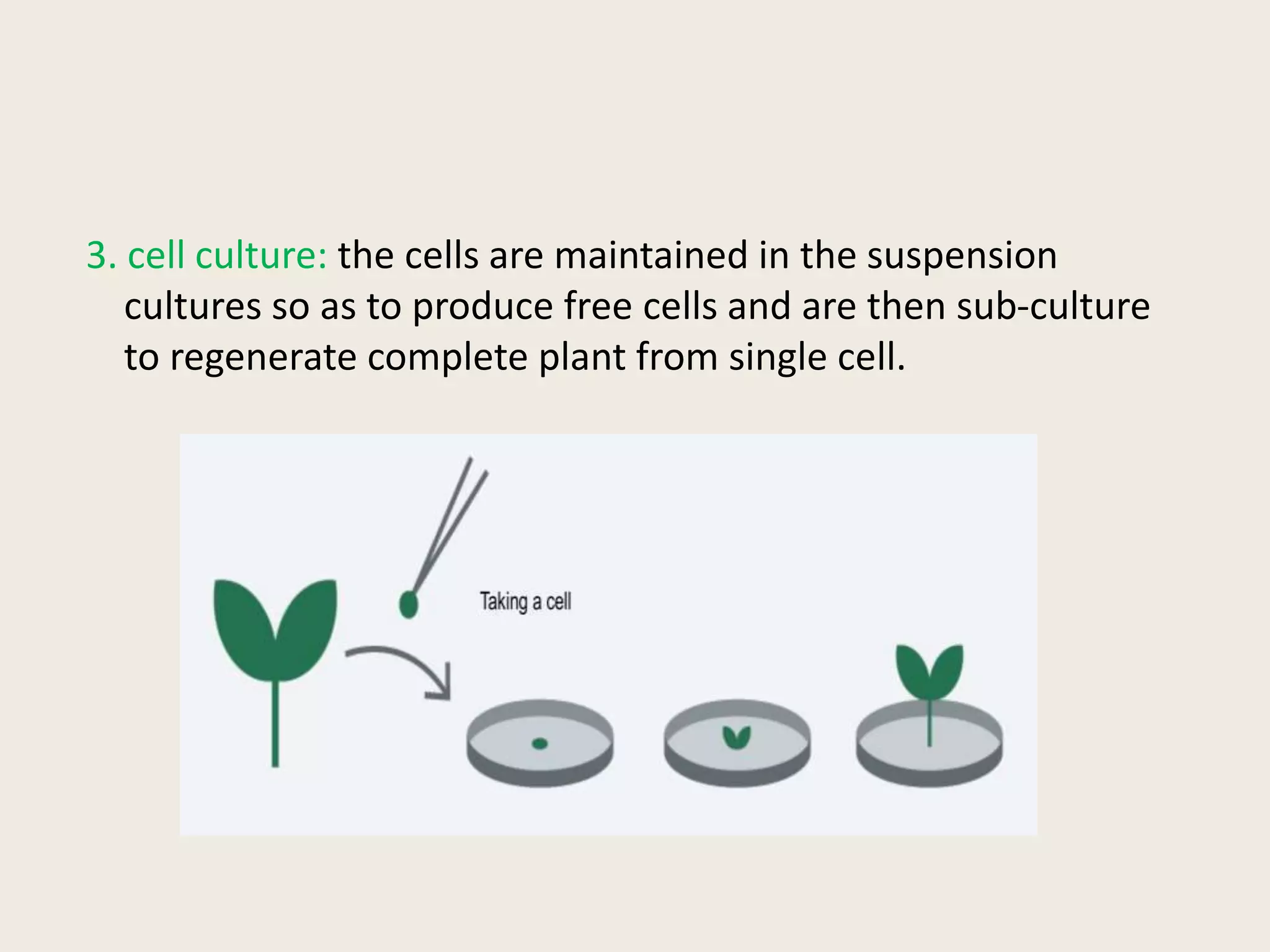
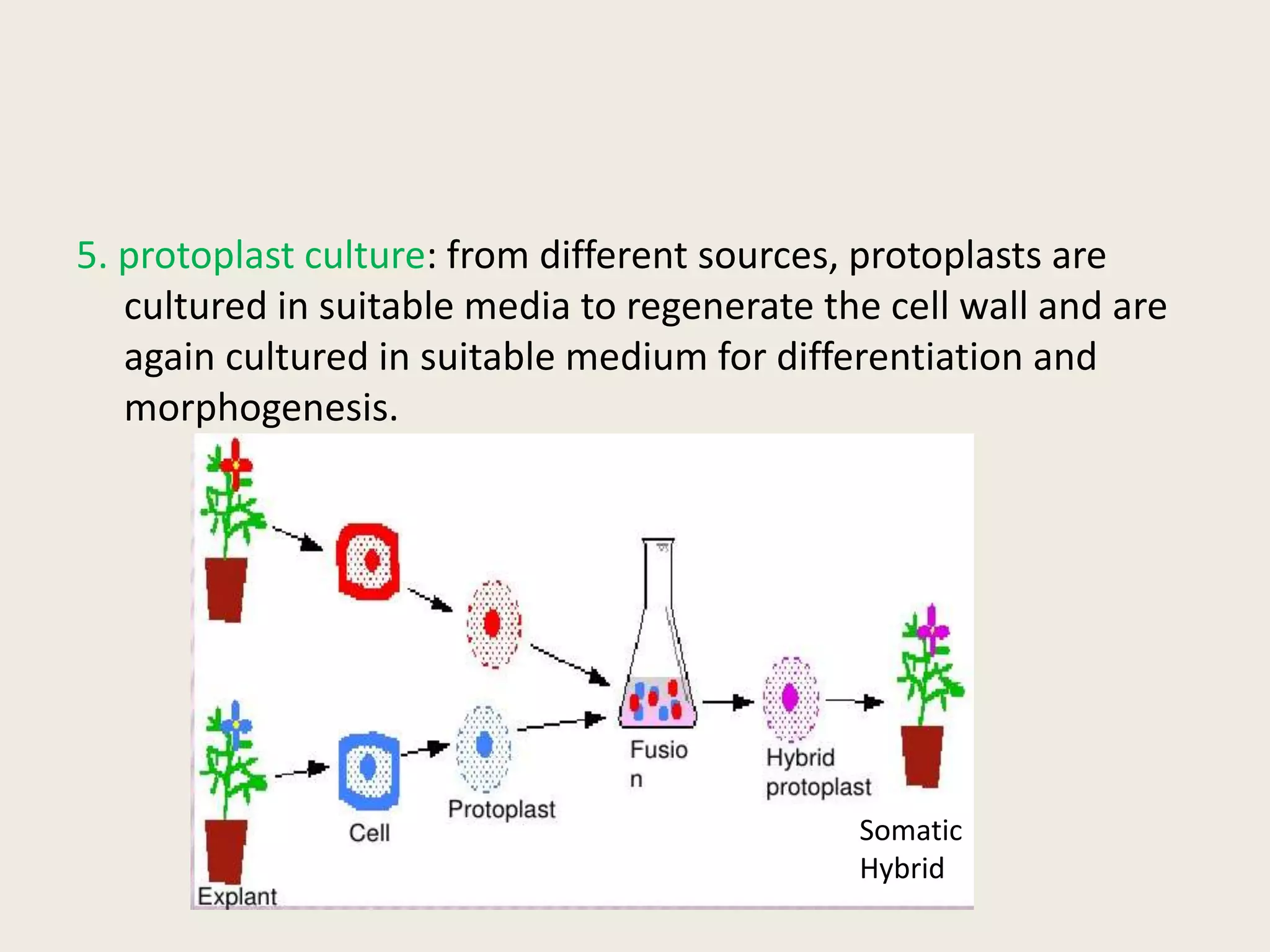
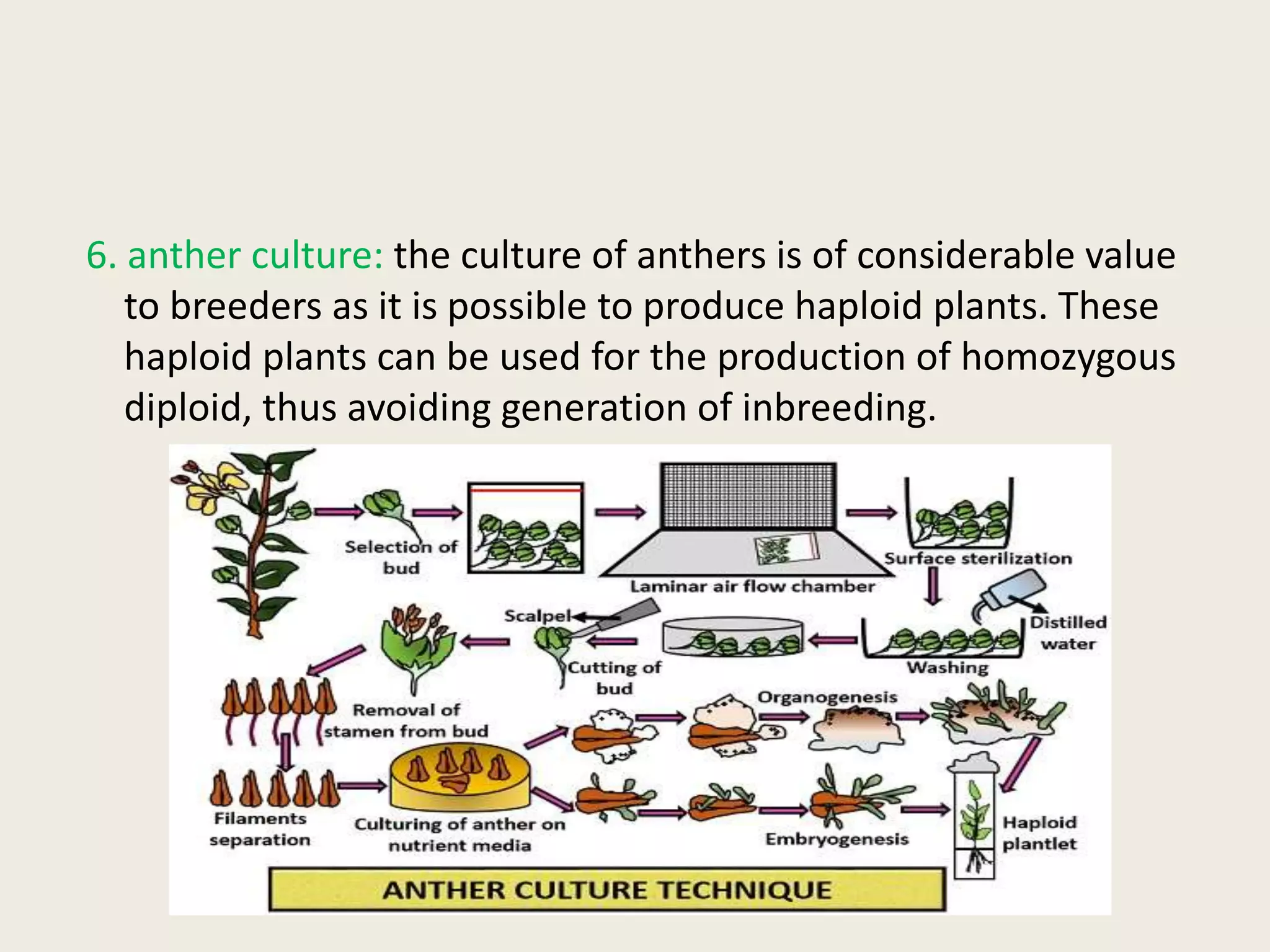
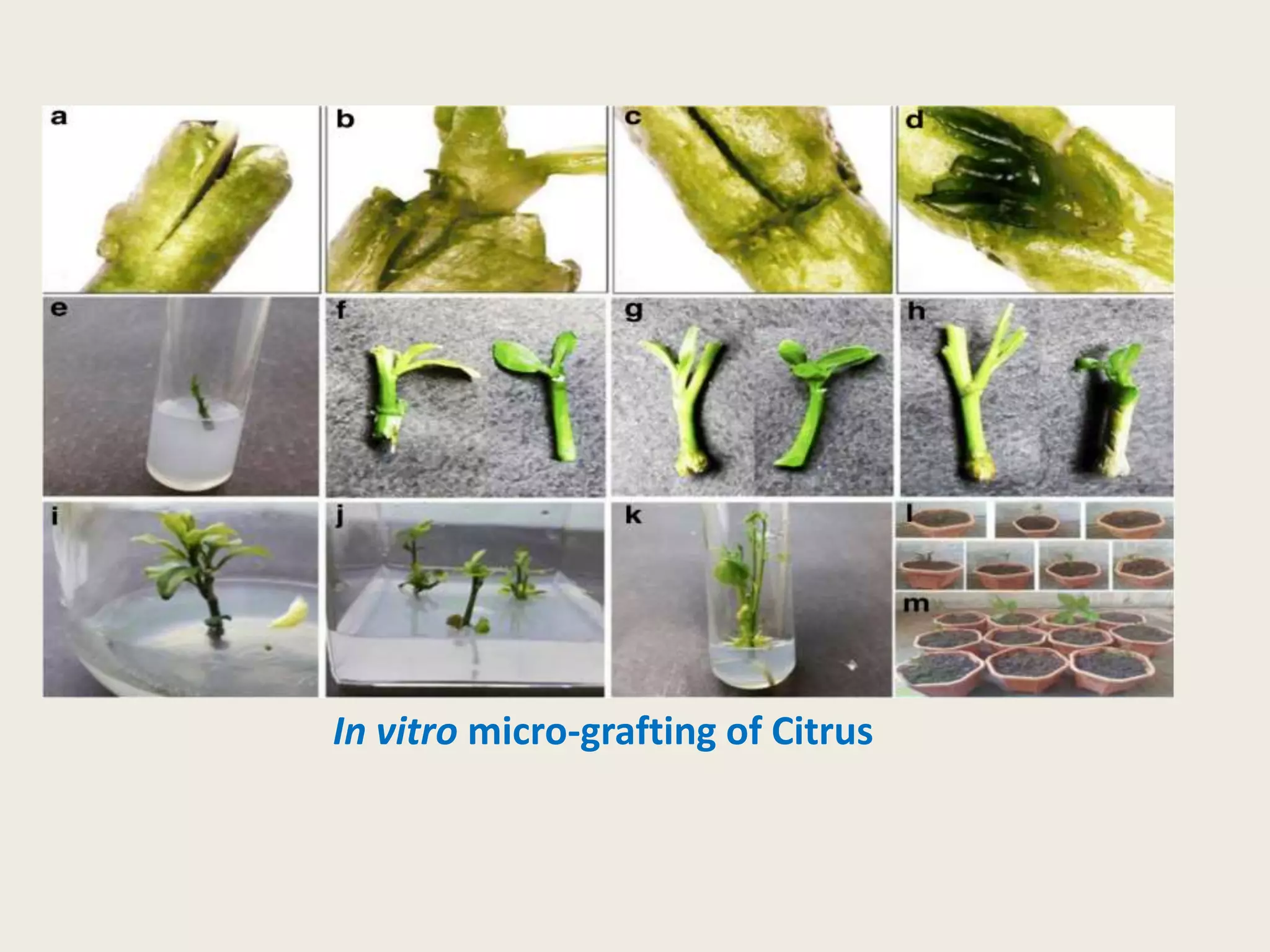
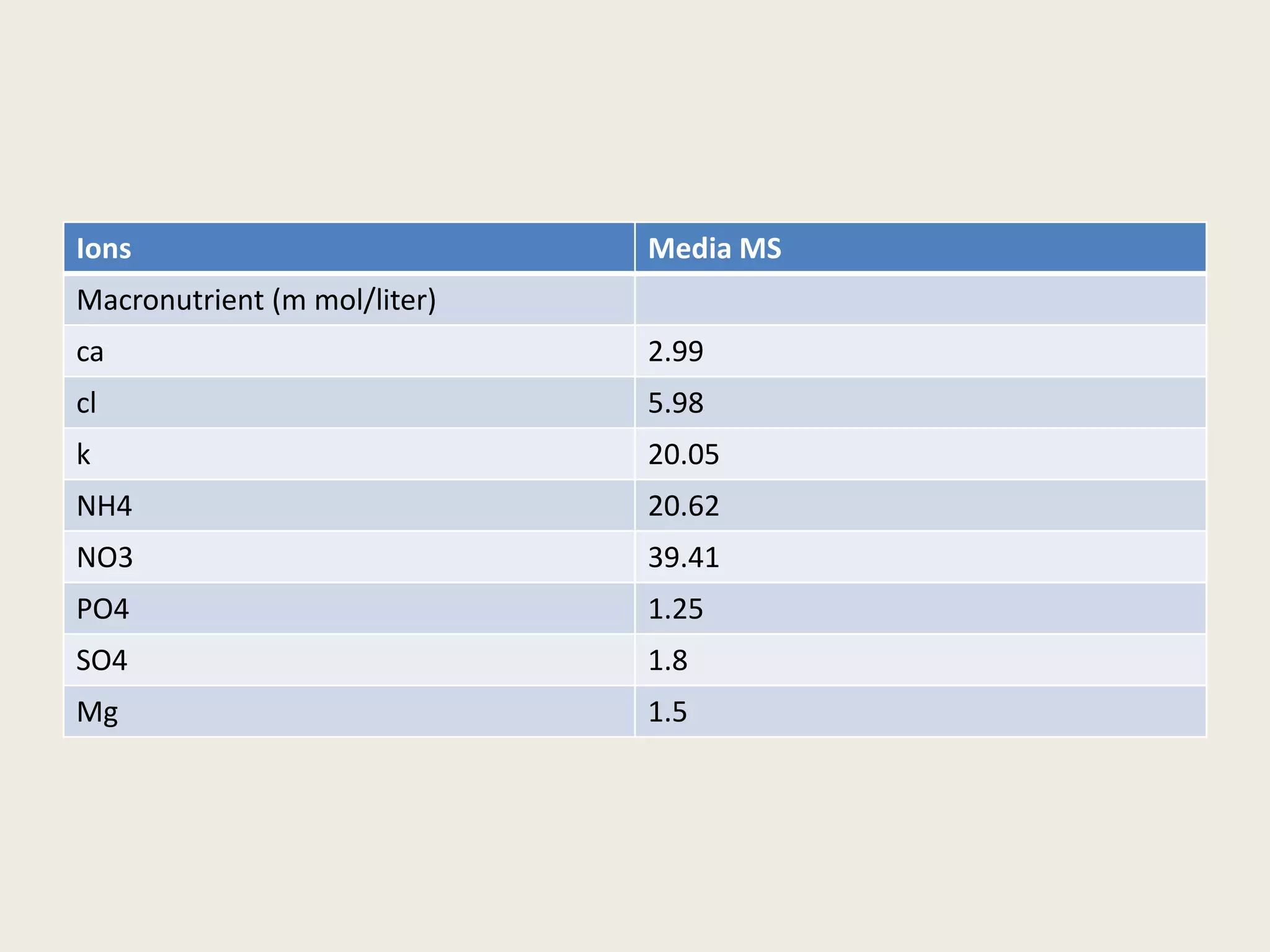
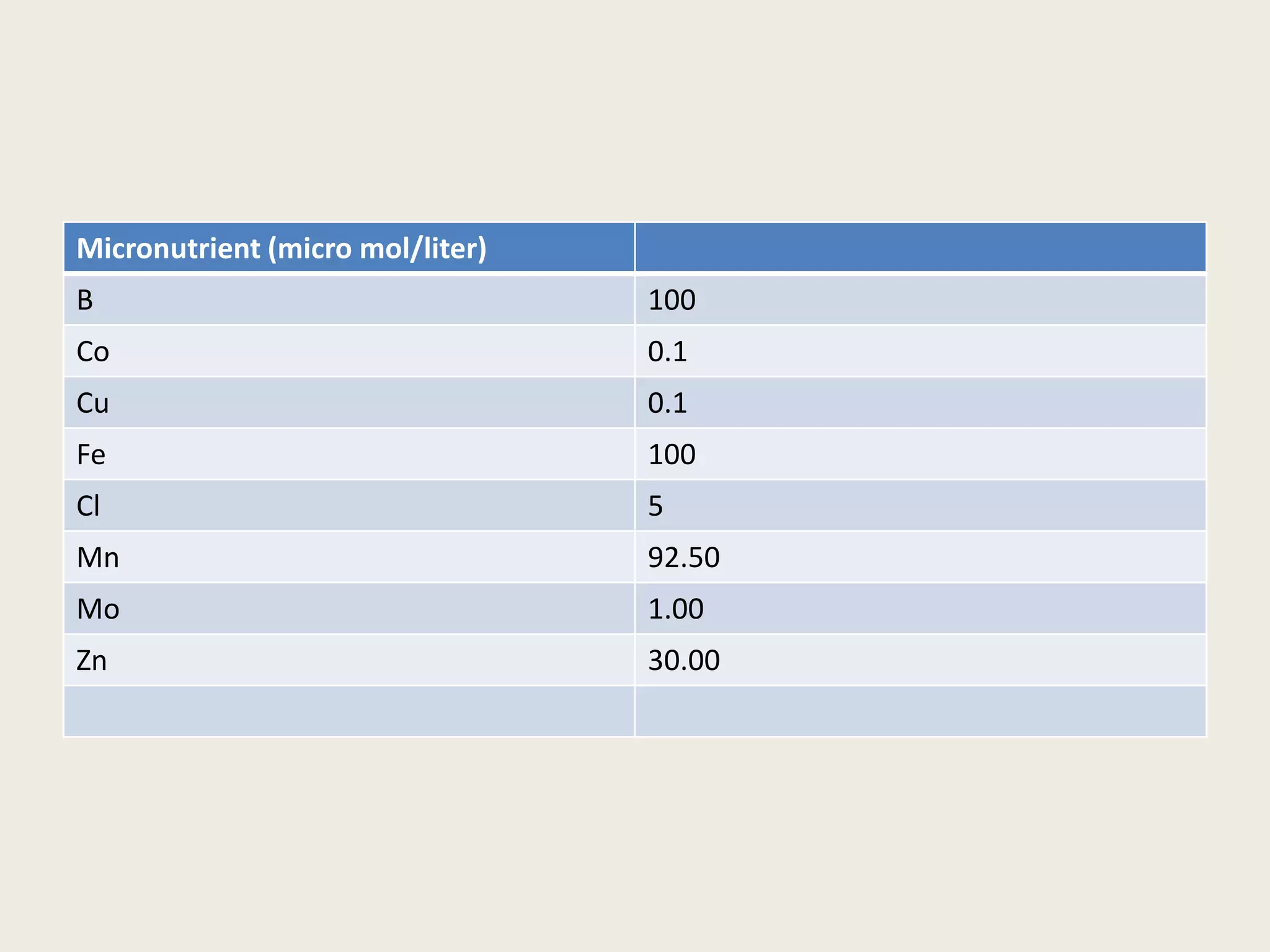
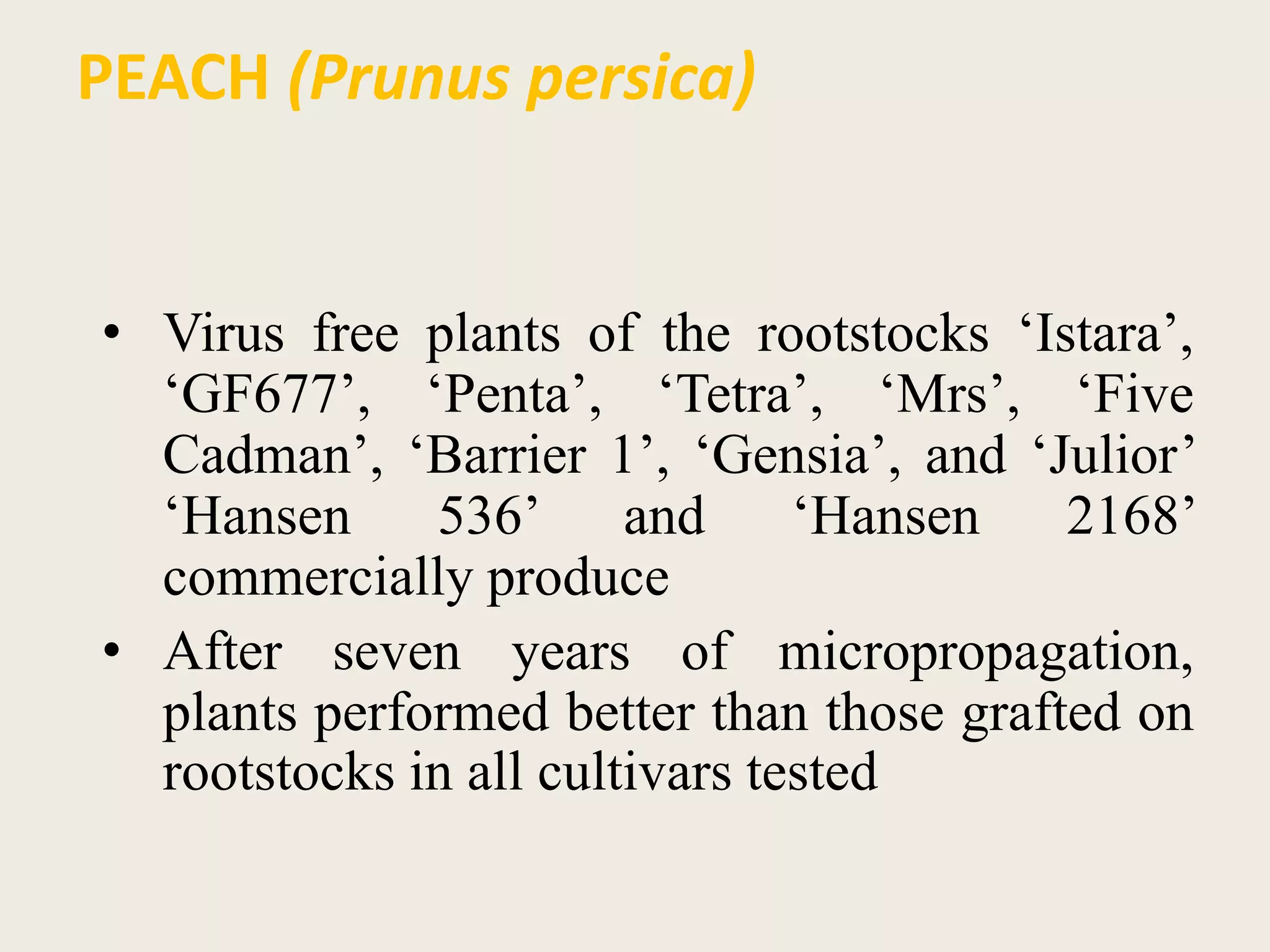
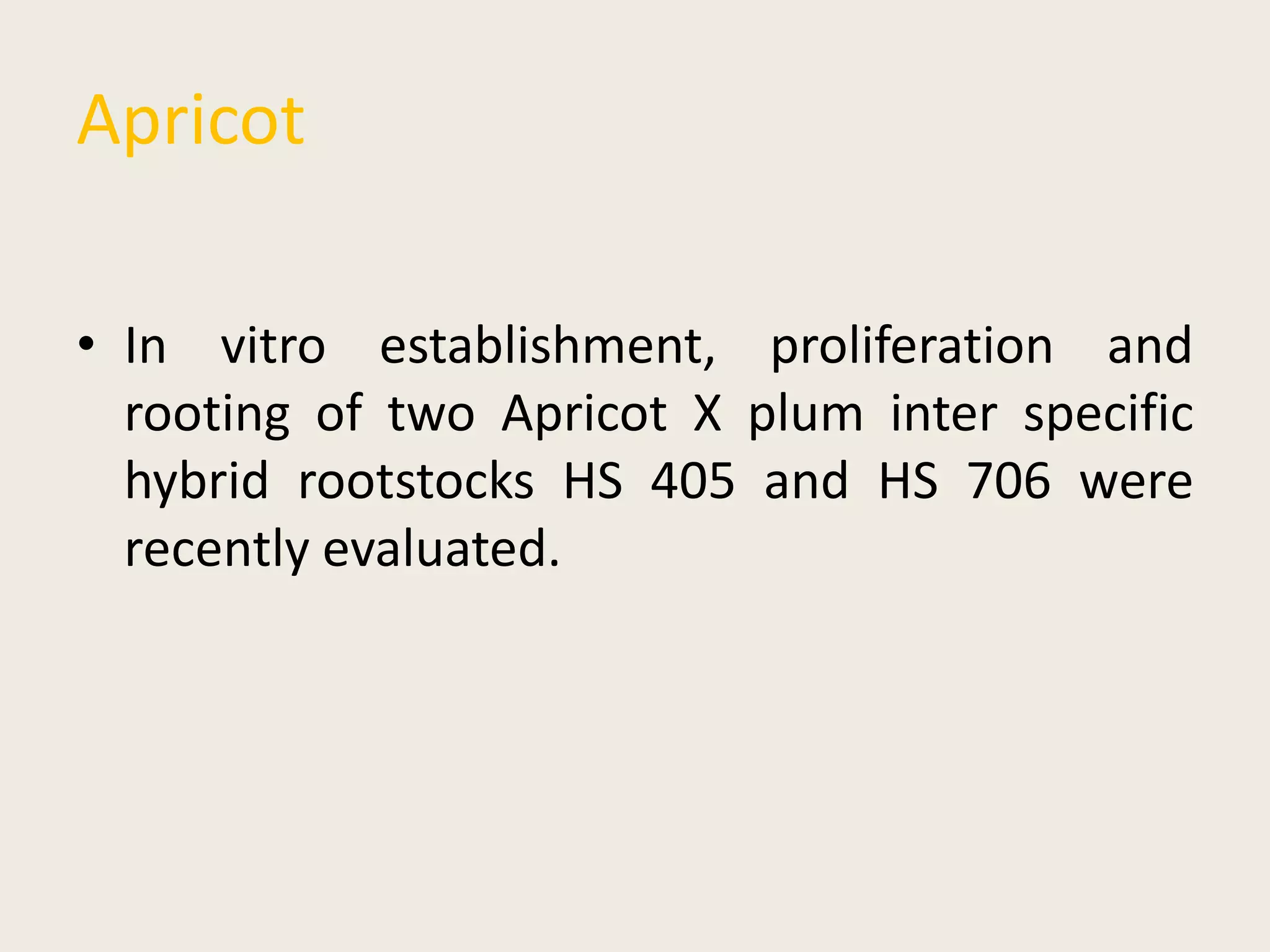
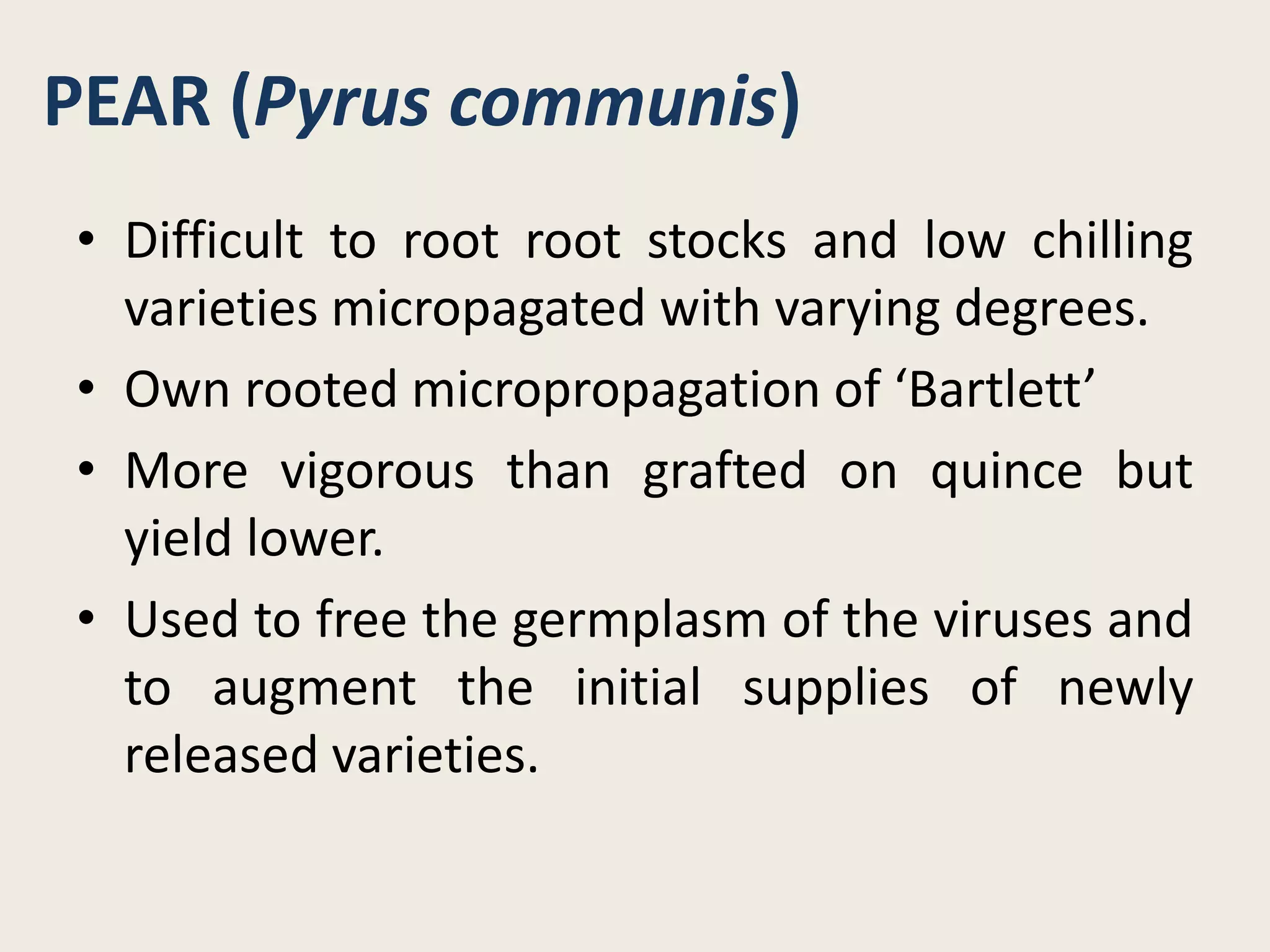
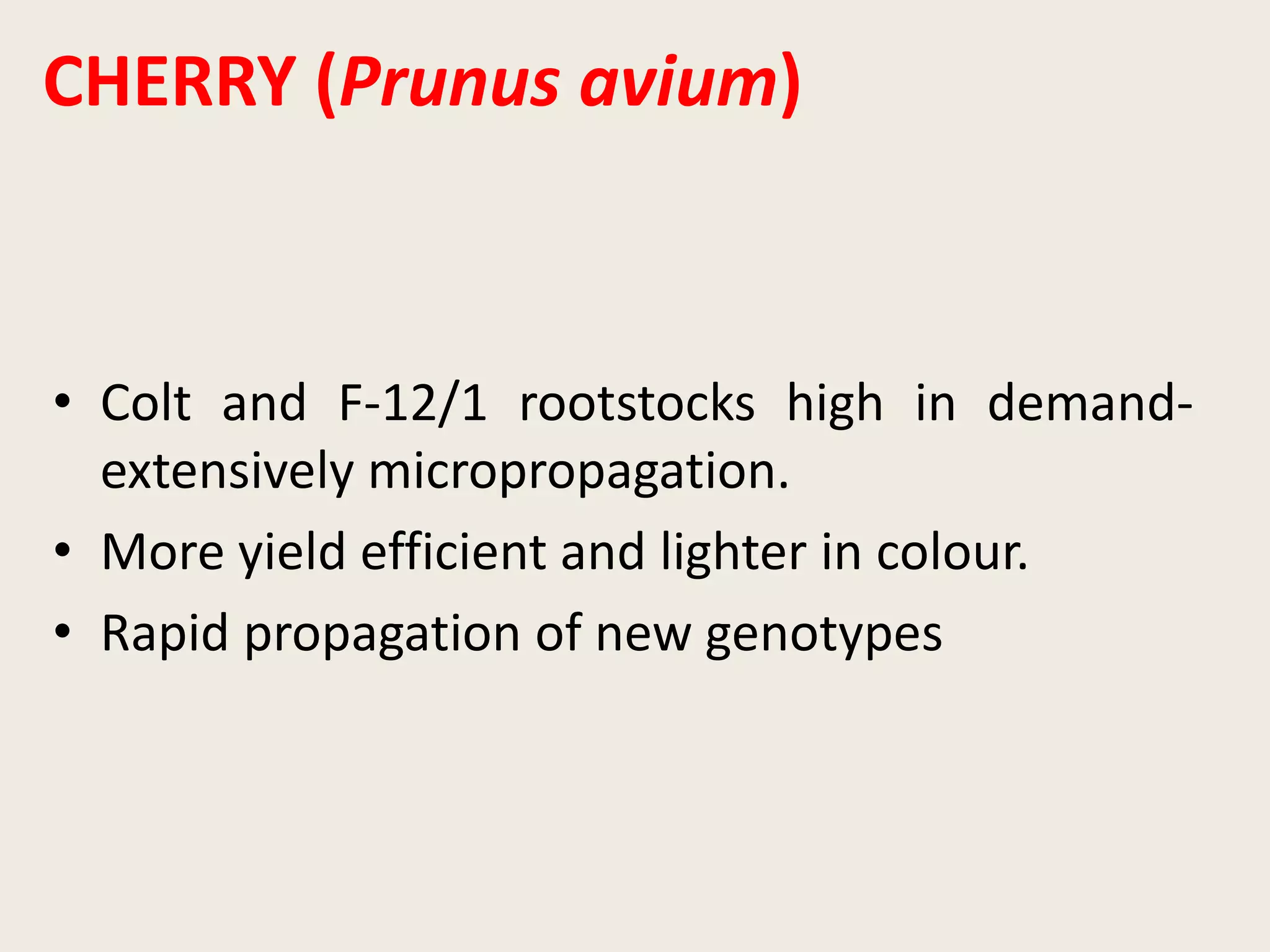
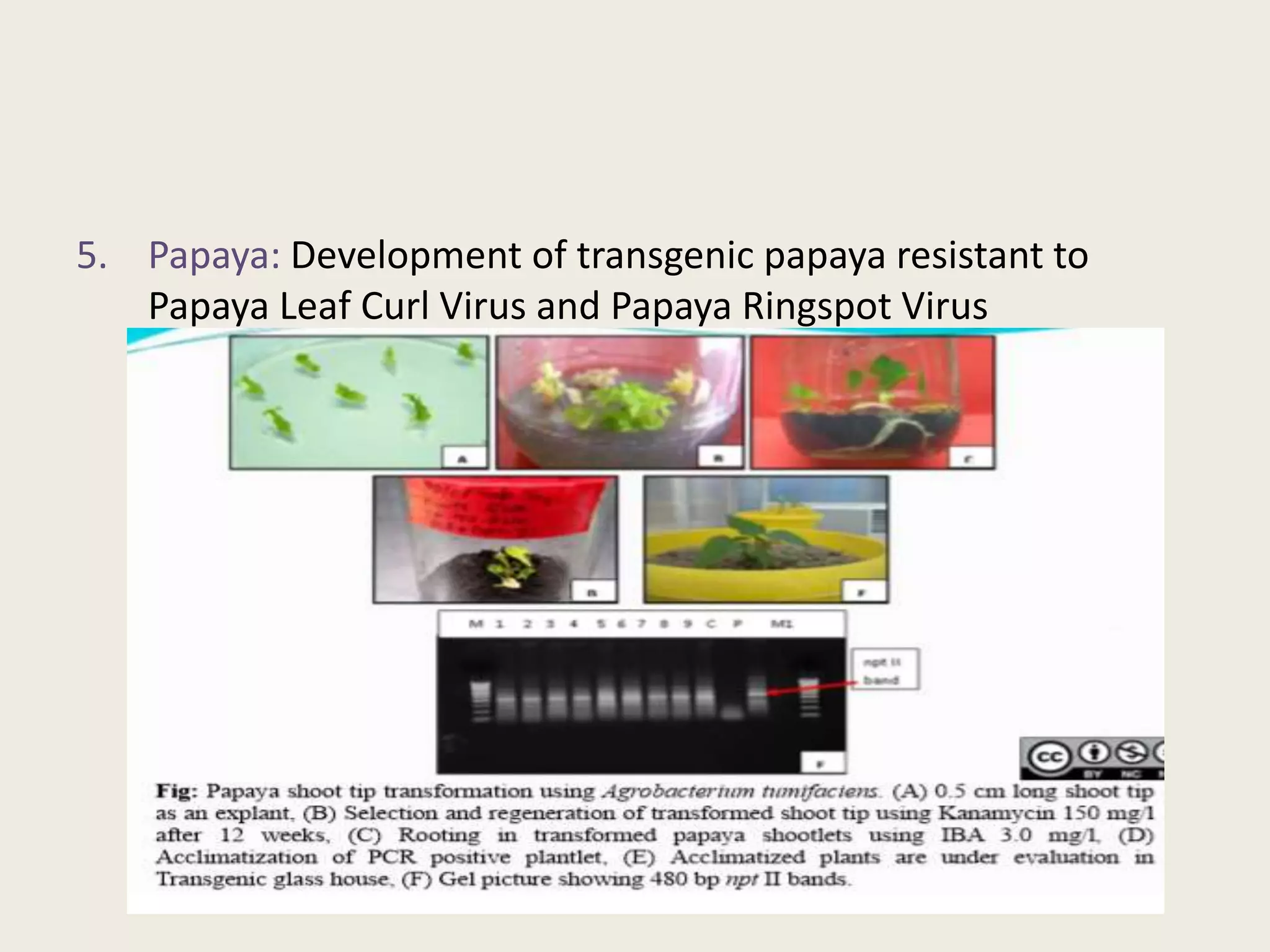
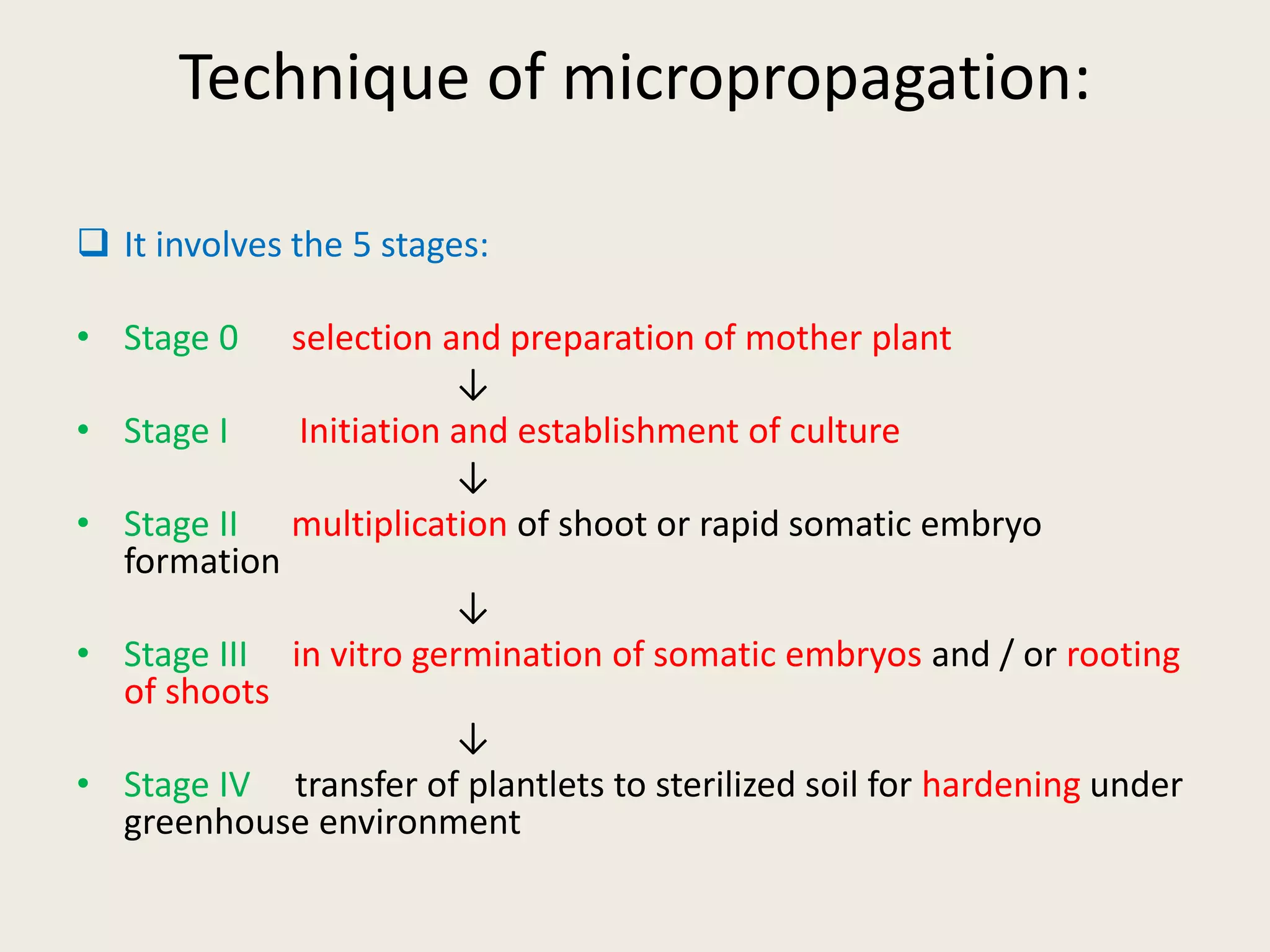
The document outlines the process of micropropagation, which involves in vitro clonal propagation of plants through tissue culture techniques. Key concepts including explant, callus, and totipotency are discussed, along with various methods of multiplication such as organogenesis and somatic embryogenesis. Applications of micropropagation, culture techniques, and stages of micropropagation are detailed, emphasizing the significance of these methods in producing disease-free plants and improving horticultural practices.