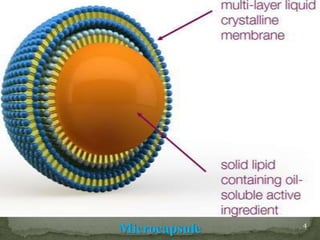
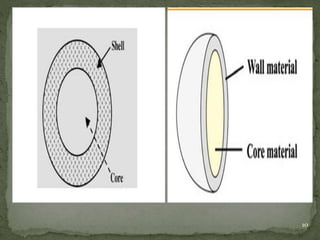
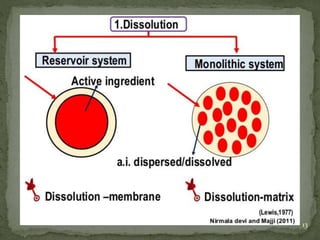
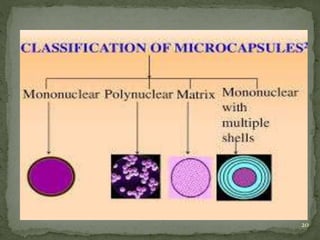
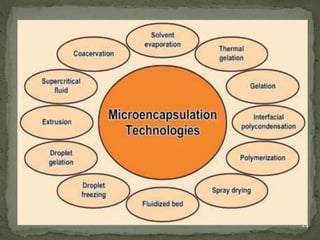
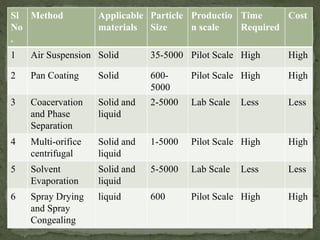
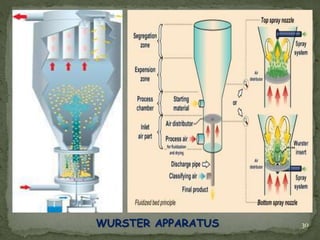
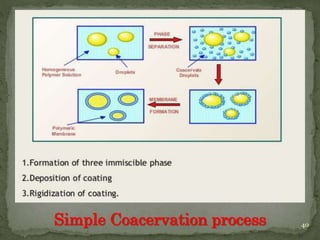
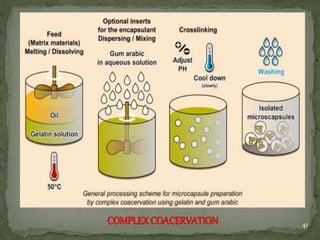
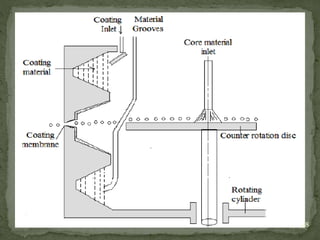
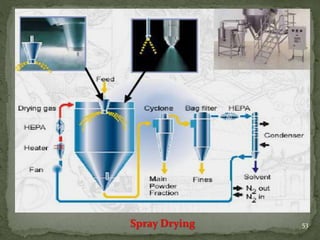
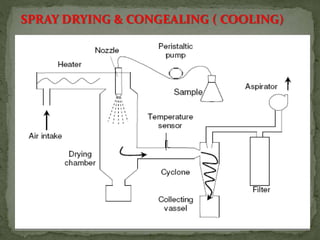
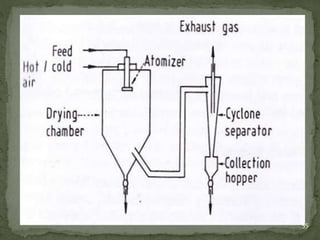
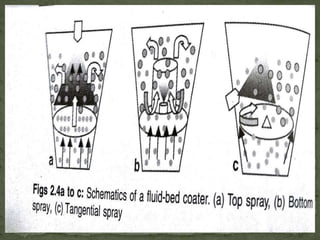
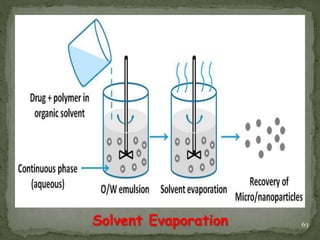
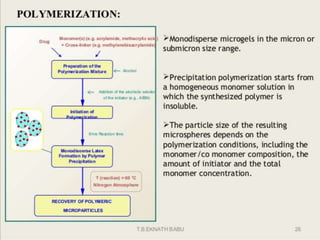
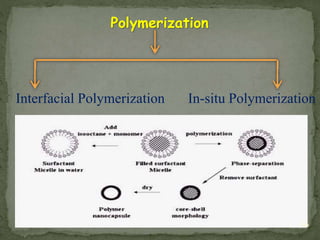
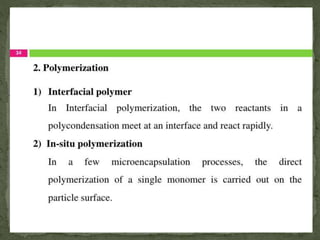
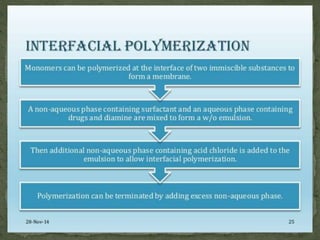
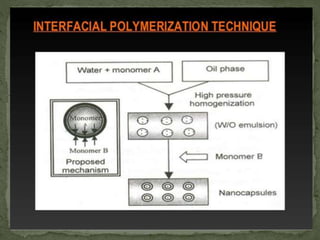
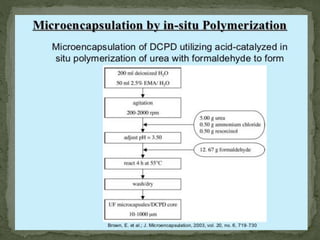
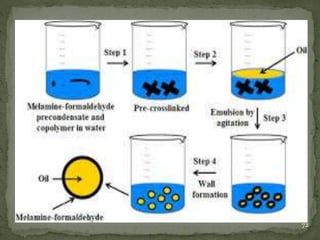
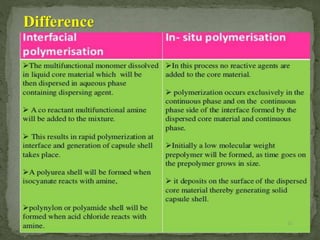
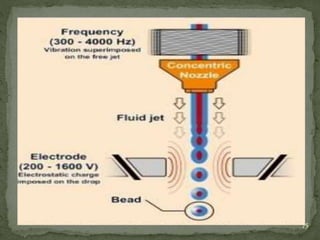
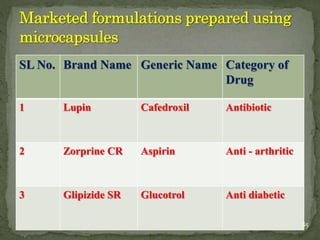
The document discusses microencapsulation and microcapsules. It defines microencapsulation as the process of coating solid or liquid core materials on a very small scale, usually 1-1000 microns in size. The core materials can be drugs, flavors, or fragrances. The coating materials are typically polymers that act as shells to provide controlled release or stabilization. Several microencapsulation methods are described in detail, including pan coating, solvent evaporation, phase separation, spray drying, and polymerization. The mechanisms of drug release from microcapsules and some applications of microencapsulation technology are also summarized.