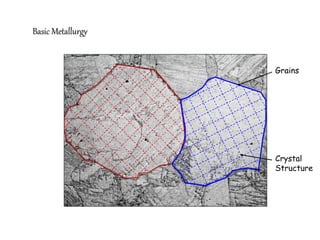
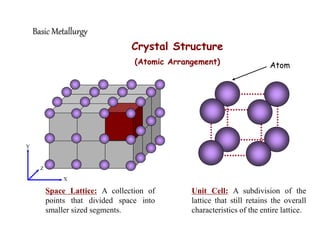
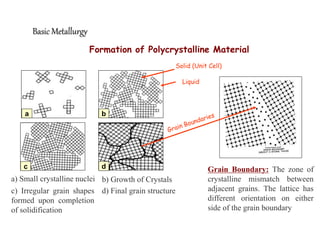
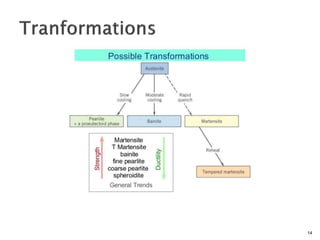
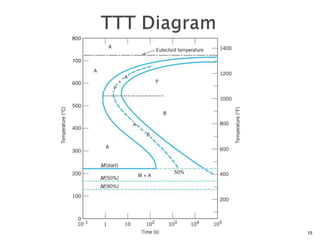
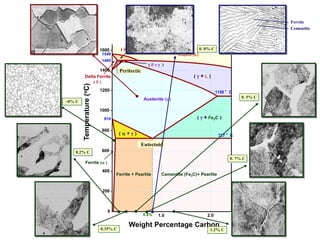
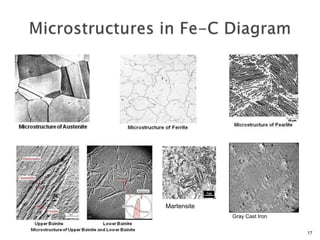
1) The document discusses the crystal structure and atomic arrangement of metals, including the space lattice, unit cell, and grain boundaries.
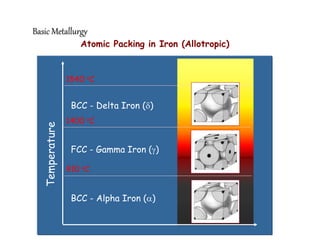
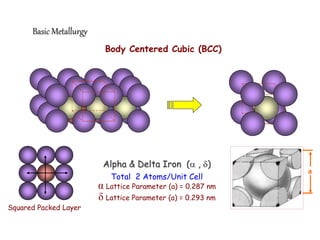
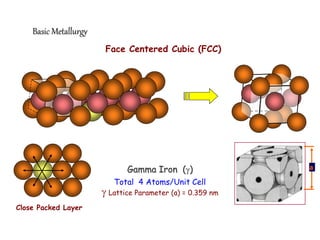
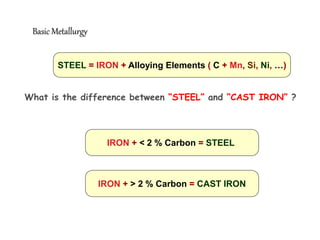
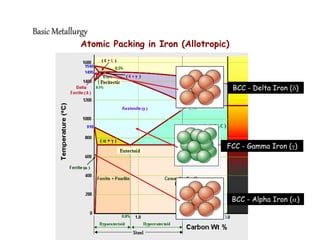
2) It describes different crystal structures like body centered cubic (BCC) and face centered cubic (FCC), providing examples for alpha, delta, and gamma iron.
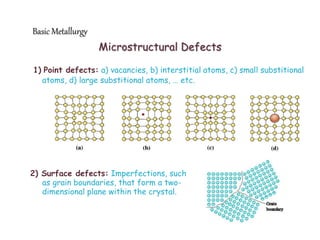
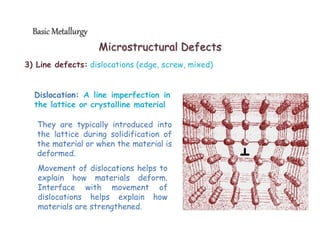
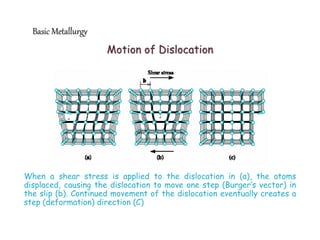
3) Various microstructural defects are outlined, including point defects, surface defects like grain boundaries, and line defects like dislocations which allow deformation through motion.