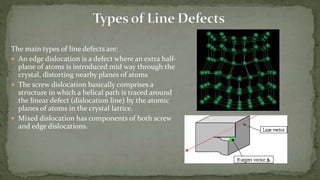
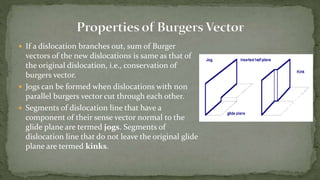
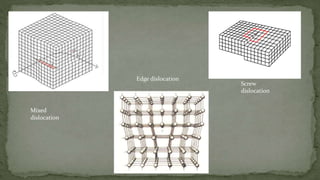
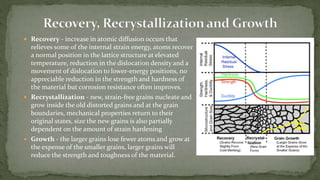
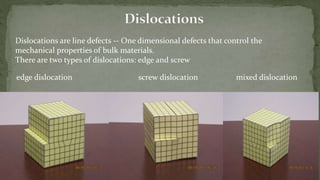
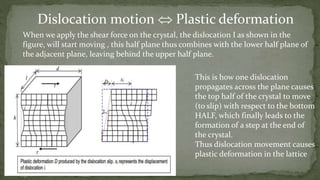
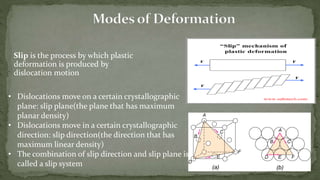
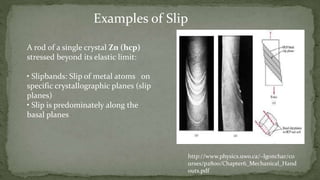
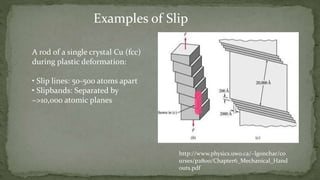
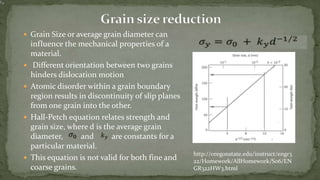
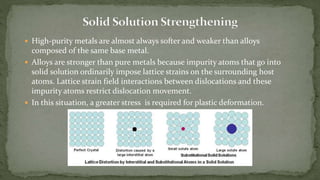
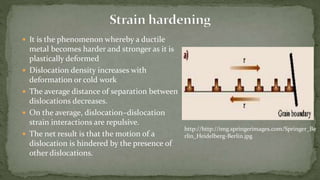
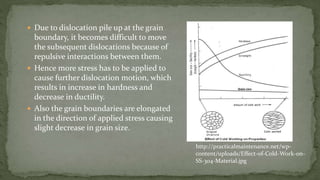
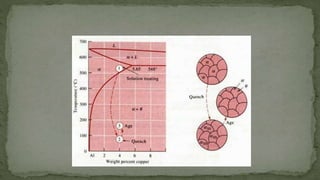
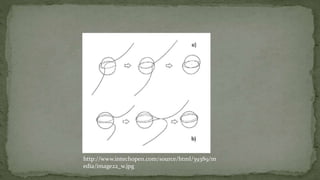
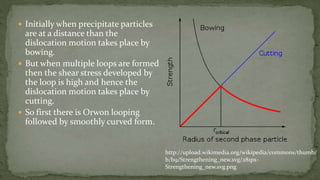
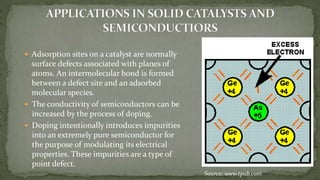
Point defects are defects that occur at a single lattice point and are not extended in space. The main types are vacancies, interstitials, and substitutions. Line defects include edge, screw, and mixed dislocations. Grain boundaries are interfaces between crystalline grains. Volume defects are 3D aggregates of atoms or vacancies that manifest as pores and cracks.