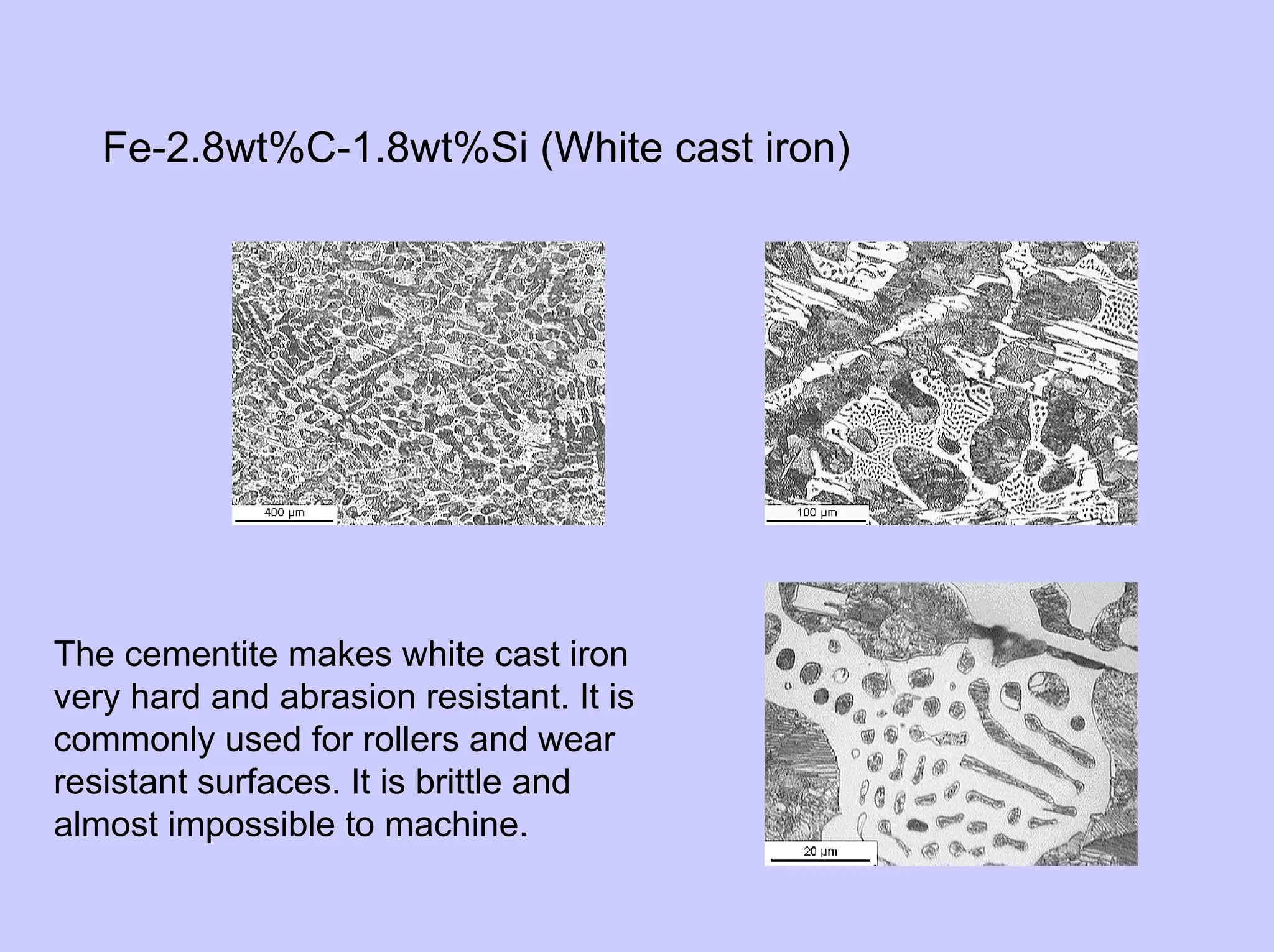
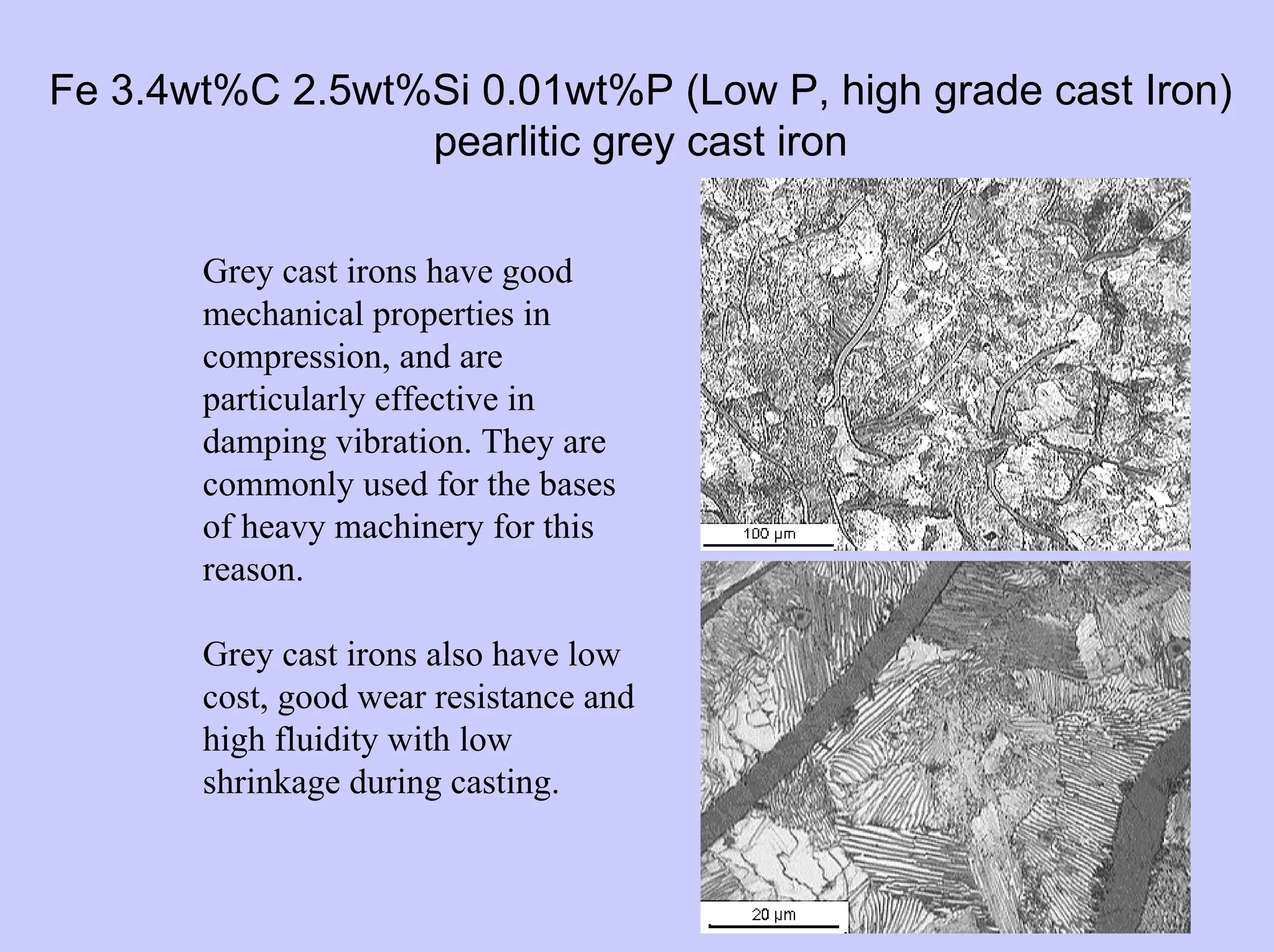
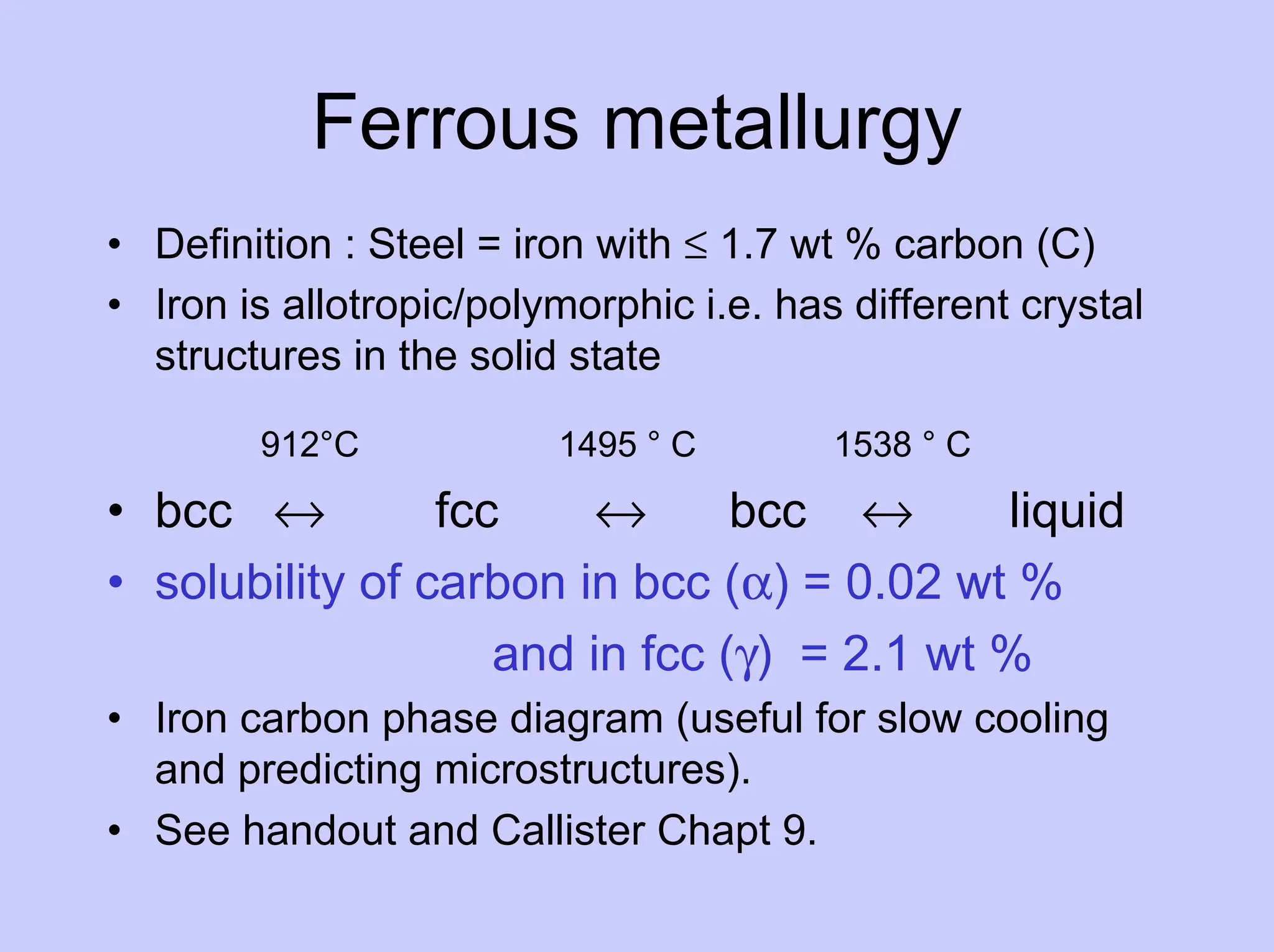
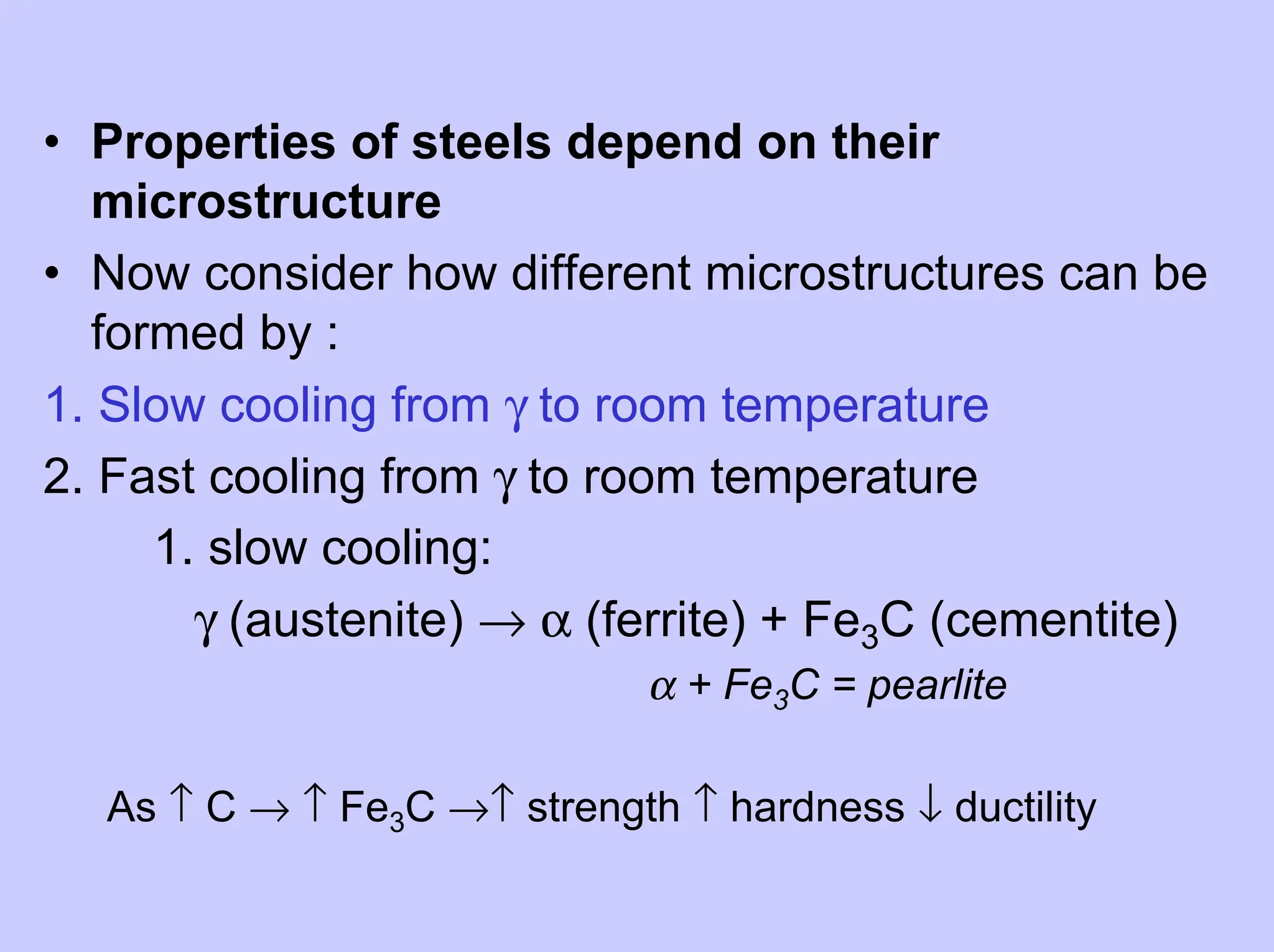
This document provides an overview of materials science as it relates to metals. It discusses strengthening mechanisms in metals like grain boundaries, solid solution hardening, work hardening, and precipitation hardening. It classifies metals into ferrous and non-ferrous alloys. For ferrous alloys like steels, it describes the iron-carbon phase diagram and how different microstructures form based on cooling rate, influencing properties. It discusses specific steel types and applications. For non-ferrous metals, it provides examples like aluminum and nickel.




![A pure metal is very soft and weak
• Because dislocations move easily
(plastic deformation)
• Physical metallurgy aims to restrict
movement of dislocations →
↑ strength and hardness
[shorthand ⊥ = dislocation]](https://image.slidesharecdn.com/materialsciences-240401034244-251d8eb7/75/material-sciences-in-civil-engineering-field-5-2048.jpg)









![Iron-carbon phase diagram
• see handout including constituents, symbols and
description [Callister Chapt. 9]](https://image.slidesharecdn.com/materialsciences-240401034244-251d8eb7/75/material-sciences-in-civil-engineering-field-18-2048.jpg)




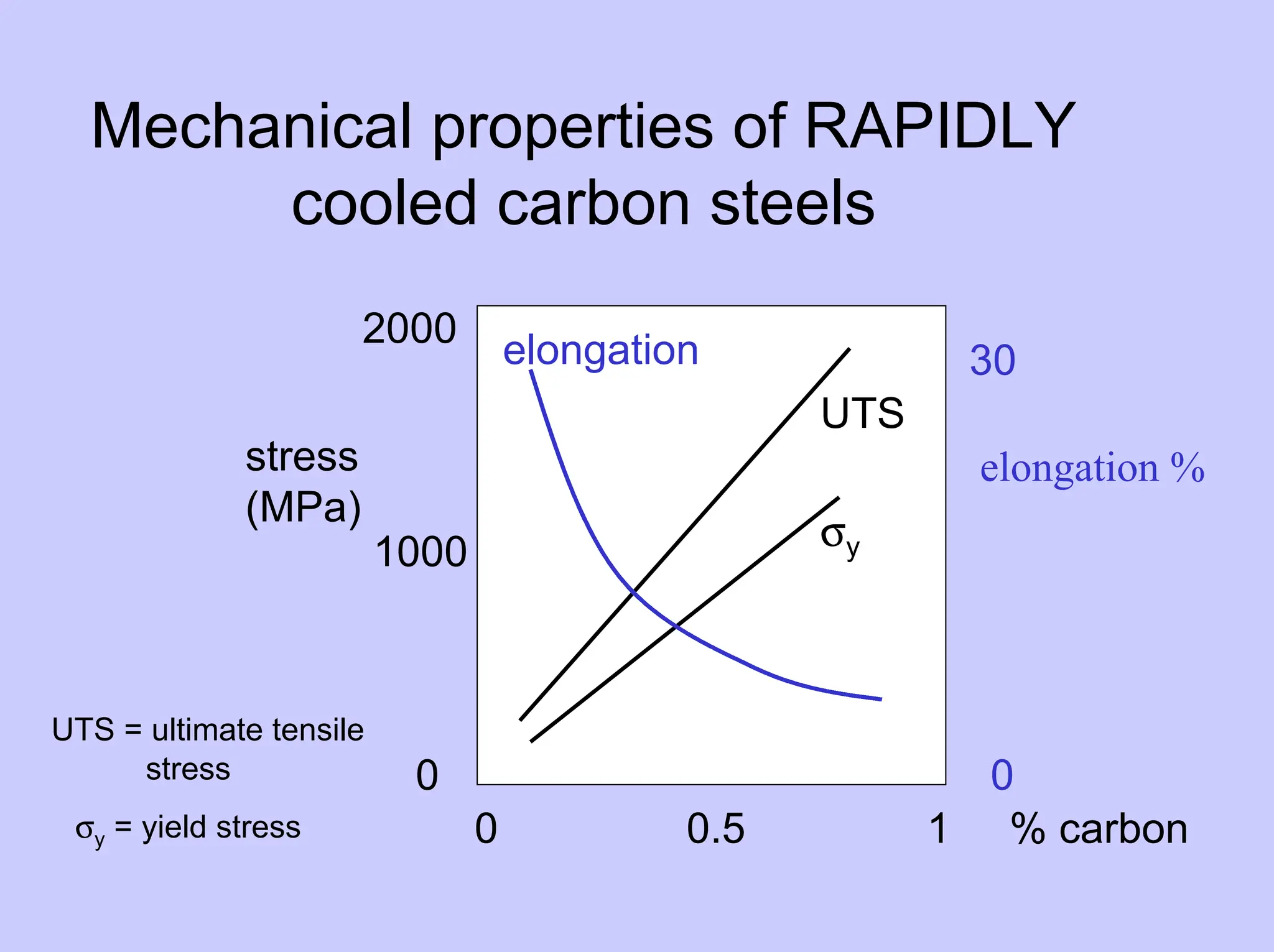
![Rapidly cooled carbon steels -
martensite
• Fast cooling from γ to room temperature
γ (austenite) → α’ (martensite)
very hard and brittle
Cooling rates required :
• Pure Fe 105 °C/s • Fe-0.8%C 200 °C/s
so α’ difficult to achieve in thick sections
and may → component distortion [Callister chpt 10]
critical
cooling
rates](https://image.slidesharecdn.com/materialsciences-240401034244-251d8eb7/75/material-sciences-in-civil-engineering-field-24-2048.jpg)