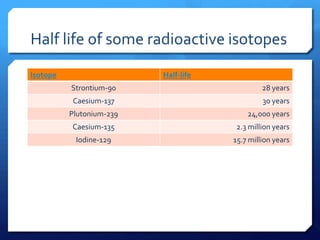
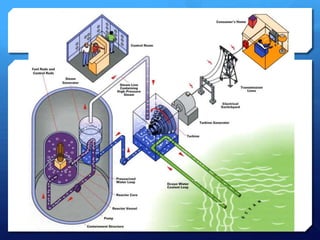
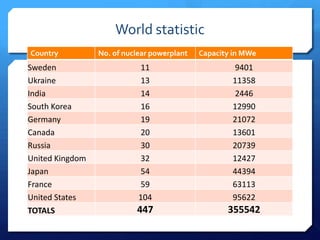
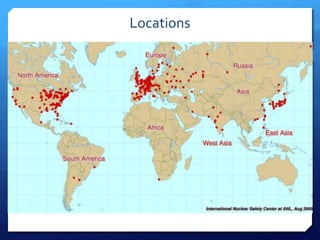
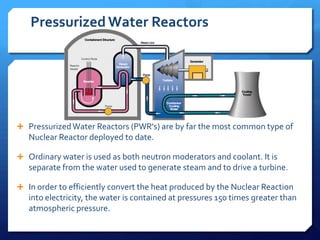
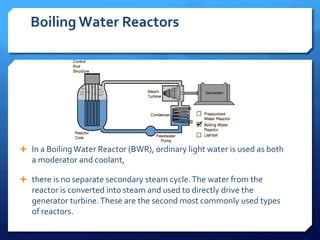
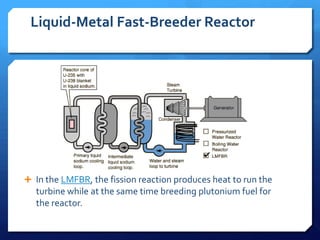
The document discusses different types of nuclear reactors used in nuclear power plants. It describes the basic process of nuclear fission and how it is used to generate heat and electricity. It then explains key components and differences between various reactor types, including pressurized water reactors, boiling water reactors, gas cooled reactors, liquid metal fast breeder reactors, and heavy water reactors. Worldwide statistics on number of plants and total capacity by country are also presented.