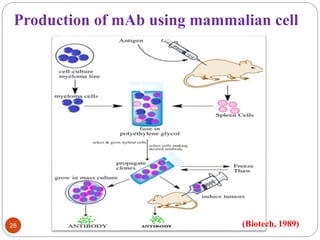
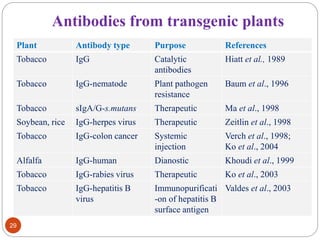
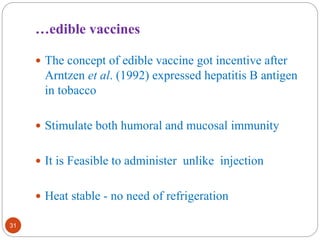
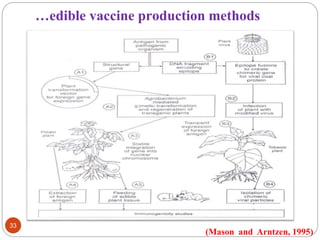
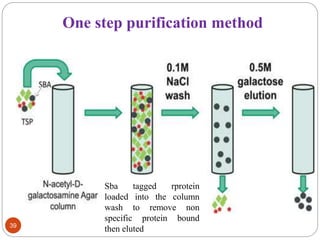
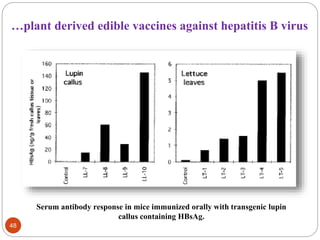
This document outlines a presentation on plant biopharming. It discusses the use of transgenic plants to produce therapeutic proteins and some key points:
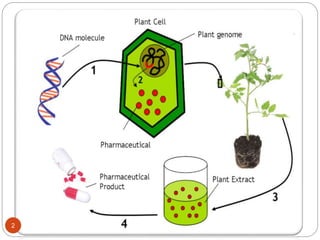
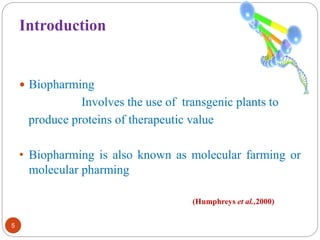
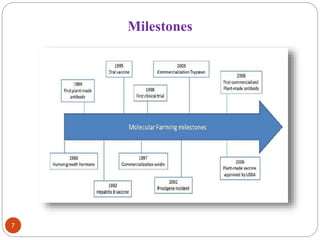
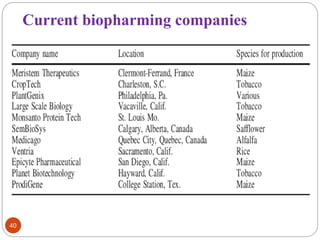
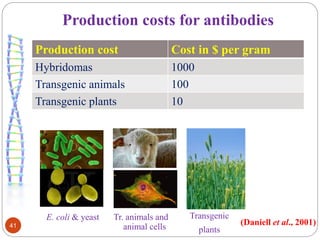
- Biopharming involves using transgenic plants to produce proteins of therapeutic value. It started about 20 years ago with the promise to produce expensive molecules cheaper.
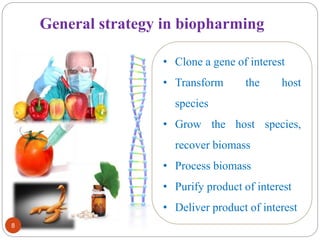
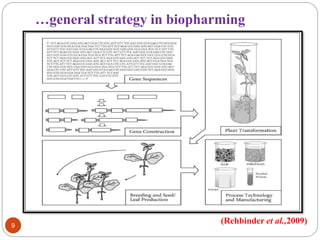
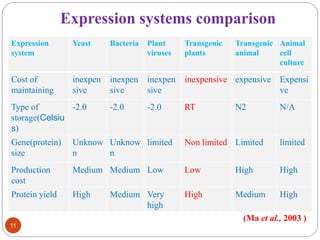
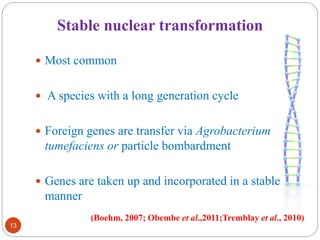
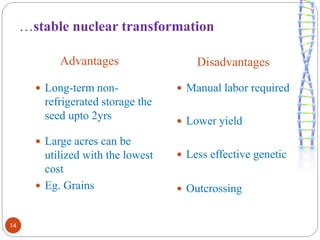
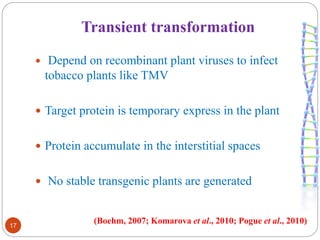
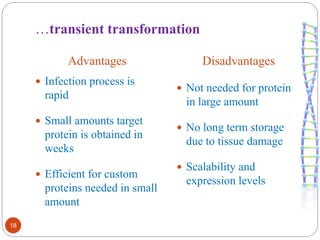
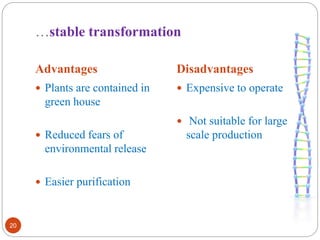
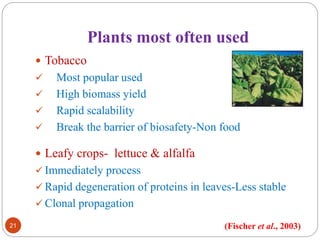
- Different production systems are discussed, including stable nuclear transformation, plastid transformation, transient transformation, and stable hydroponic transformation. Tobacco, lettuce, alfalfa, rice and maize are common plant species used.
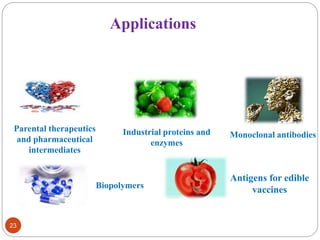
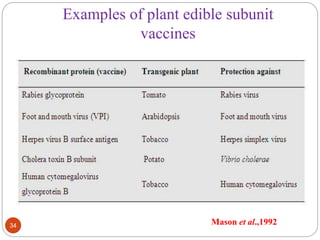
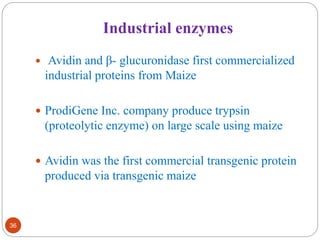
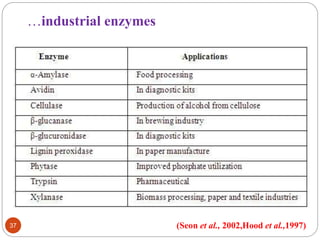
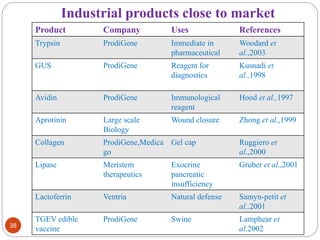
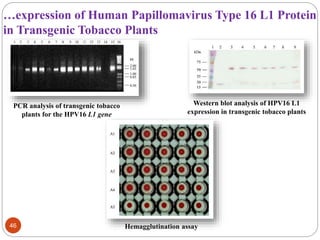
- Applications include pharmaceuticals, industrial enzymes, monoclonal antibodies, edible vaccines. Successful reports demonstrate production of measles virus protein in transgenic carrot and human papillomavirus protein