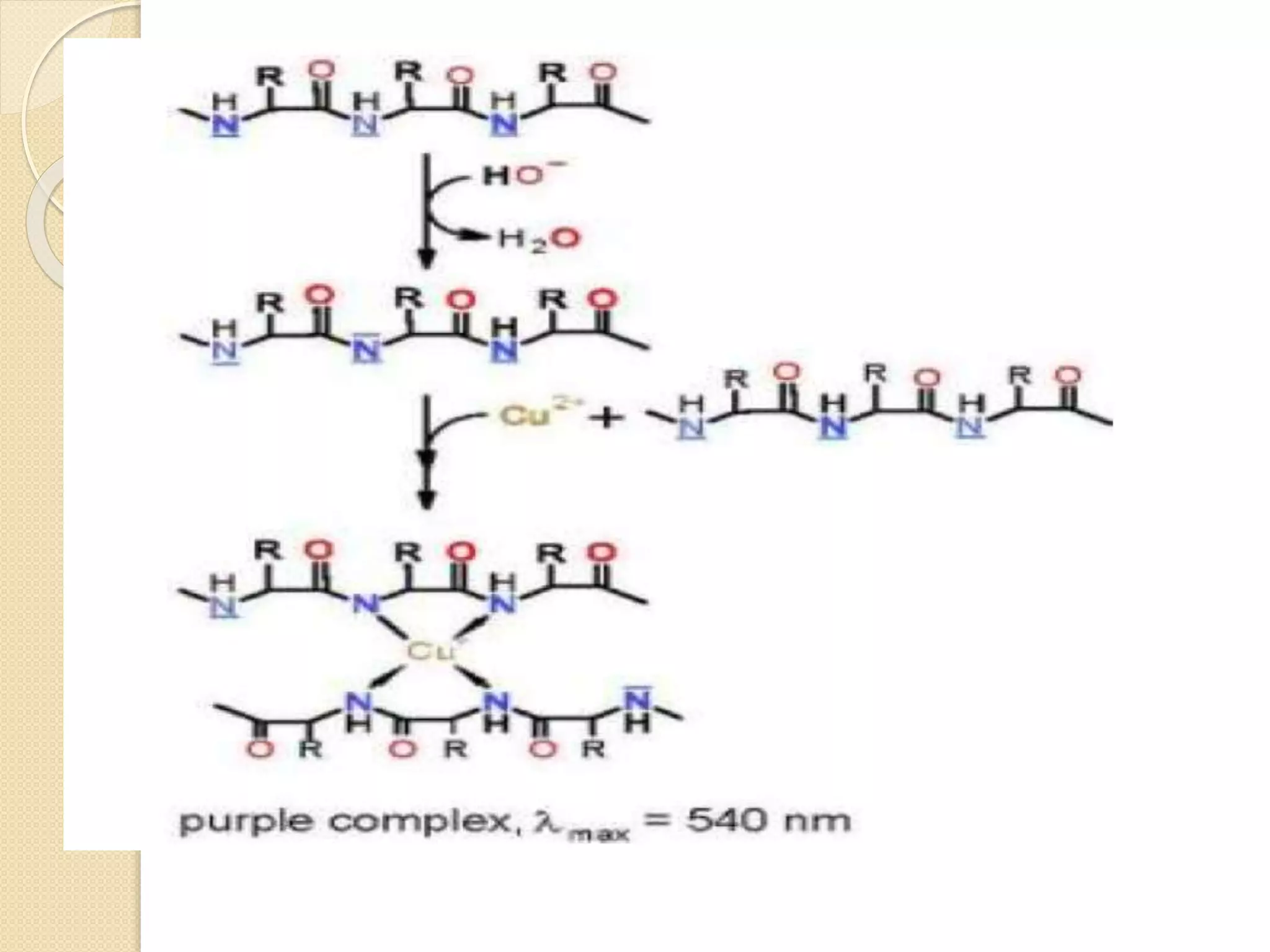
The document outlines various methods for estimating protein concentration in serum, including biuret, dye-binding, Folin-Ciocalteu (Lowry), Kjeldahl, refractometry, turbidity, and nephelometry. It details the principles, procedures, and interpretation of results for these methods, emphasizing the importance of protein measurement in biochemistry. The biuret test is highlighted as a general test for proteins, while methods like dye-binding and Folin-Ciocalteu are noted for their specific applications and reaction mechanisms.






![TITRATION
Reaction with Hydrochloric Acid (HCl): [Back
titration]
The ammonia is captured by a, carefully measured
excess of a standardized acid solution in the receiving
flask. The excess of acid in the receiving solution keeps
the pH low, and the indicator does not change color.
NH3 + HCl (in excess) = NH4+Cl- + HCl (left back)
The excess acid solution is exactly neutralized by a
carefully
measured standardized alkaline base solution such as
sodium hydroxide. A color change is produced at the
end point of the titration.
HCl + NaOH = NaCl + H2O
pg 7
V ml of X (N) NaOH = V ml of X (N) HCl = V ml of X (N)
NH3](https://image.slidesharecdn.com/presentation1-200213145931/75/serum-protein-estimation-12-2048.jpg)